Physical World/Chemistry
Chemical elements
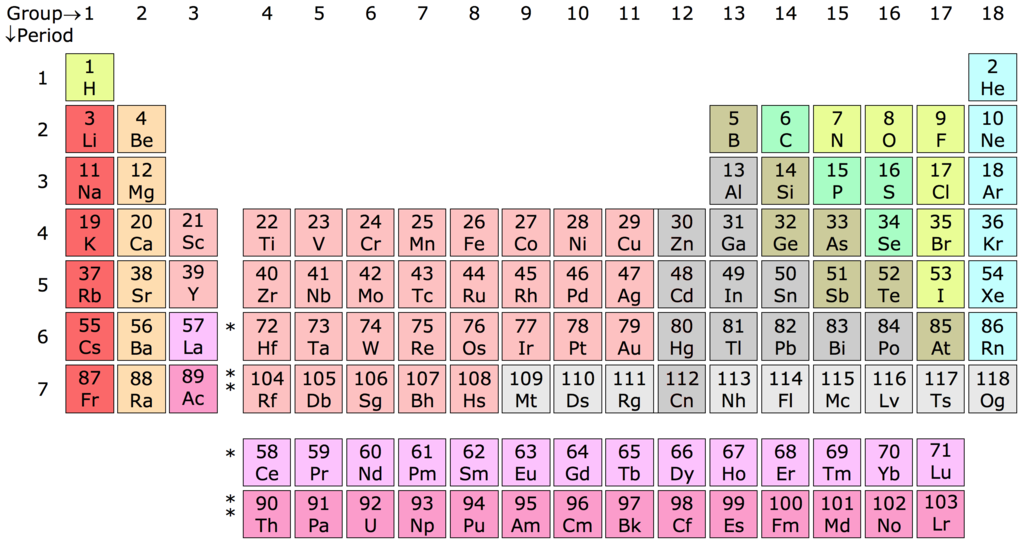
Periodic table
Dobereiner's Triads are groups of three elements having similar properties. They are named after the German chemist Johann Dobereiner. He observed that when three elements of any particular triad were arranged in order of their increasing atomic masses, the atomic mass of the middle element was roughly the mean or average of the atomic masses of the other two elements. He was the first to classify elements into groups based on John Dalton's assertions
John Newlands prepared in 1863 the first periodic table of the elements arranged in order of relative atomic masses, and pointed out in 1865 the 'law of octaves' whereby every eighth element has similar properties
Invention of the periodic table is generally credited to Russian chemist Dmitri Mendeleev in 1869
To give provisional names to his predicted elements, Mendeleev used the prefixes eka-, dvi-, and tri-, from the Sanskrit names of digits 1, 2, and 3, depending upon whether the predicted element was one, two, or three places down from the known element of the same group in his table
The four predicted elements lighter than the rare earth elements, ekaboron (Eb), ekaaluminium (Ea), ekamanganese (Em), and ekasilicon (Es), proved to be good predictors of the properties of scandium, gallium, technetium and germanium respectively, which each fill the spot in the periodic table assigned by Mendeleev
Antonius van den Broek was the first person to realize that the number of an element in the periodic table (now called atomic number) corresponds to the charge of its atomic nucleus. This hypothesis was published in 1911
Moseley's law is an empirical law concerning the characteristic x-rays that are emitted by atoms. The law was invented and published by the English physicist Henry Moseley in 1913. It is historically important in quantitatively justifying the conception of the nuclear model of the atom, with all, or nearly all, positive charges of the atom located in the nucleus, and associated on an integer basis with atomic number. Until Moseley's work, ‘atomic number’ was merely an element's place in the periodic table, and was not known to be associated with any measureable physical quantity
Atomic number (also known as the proton number) is the number of protons found in the nucleus of an atom. It is traditionally represented by the symbol Z. The atomic number uniquely identifies a chemical element. In an atom of neutral charge, the number of electrons also equals the atomic number. The atomic number is closely related to the mass number (A), which is the number of protons and neutrons in the nucleus of an atom
The mass number is written either after the element name or as a superscript to the left of an element's symbol. For example, the most common isotope of carbon is carbon-12, or 12C, which has 6 protons and 6 neutrons
Relative atomic mass of an element takes into account the relative abundance of each isotope. Also known as atomic weight and molecular mass
Atomic mass unit – one twelfth of the rest mass of an unbound atom of carbon-12 in its nuclear and electronic ground state, and has a value of 1.6605×10−27 kg
Jons Jacob Berzelius compiled a table of relative atomic weights. This work provided evidence in favour of the atomic theory proposed by John Dalton. He developed a system of chemical notation in which the elements were given simple written labels
Five main points of John Dalton's atomic theory:
1. The atoms of a given element are different from those of any other element; the atoms of different elements can be distinguished from one another by their respective relative atomic weights
2. All atoms of a given element are identical
3. Atoms of one element can combine with atoms of other elements to form chemical compounds; a given compound always has the same relative numbers of types of atoms
4. Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process; a chemical reaction simply changes the way atoms are grouped together
5. Elements are made of tiny particles called atoms
Allotrope – two or more forms of an element due to different arrangement of atoms
Isotopes – any of the several different forms of an element each having different atomic mass (mass number). Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. Therefore, isotopes have different mass numbers, which give the total number of nucleons – the number of protons plus neutrons
Elements by group
Group – also known as a family, is a vertical column in the periodic table of the elements. Elements in a group have the same number of electrons in their outermost shell
Period – a horizontal row in the periodic table of the elements. The period number indicates how many electron shells the elements in that period have. The electron shells are filled from the innermost shell outwards
Atomic radii generally decrease along each period (row) of the table, from the alkali metals to the noble gases; and increase down each group (column). The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period
Ionization energy increases from left to right in a period and decreases from top to bottom in a group
Block – a set of adjacent groups. The respective highest-energy electrons in each element in a block belong to the same atomic orbital type. Each block is named after its characteristic orbital; thus, the blocks are: s-block, p-block, d-block, and f-block
Electron configuration – the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For the d-block subtract shell number by 1, For the f-block subtract by 2
Rare earth elements or rare earth metals are a set of seventeen chemical elements in the periodic table, specifically the fifteen lanthanoids plus scandium and yttrium. The word ‘earth’ refers to metal oxides
Lanthanides (also known as lanthanoids) are rare earth elements – elements 57 to 71. All lanthanides are f-block elements in Period 6, corresponding to the filling of the 4f electron shell
Most lanthanides were discovered in the minerals cerite and gadolinite
Actinides (also known as actinoids) – elements 89 to 103, f-block elements in Period 7
Transactinide elements are the chemical elements with atomic numbers greater than those of the actinides, the heaviest of which is lawrencium (103). The transactinide elements (104–118) are also called super-heavy elements. All transactinide elements are also transuranic elements (93–118), that is, have an atomic number greater than that of uranium (92), an actinide
Group 1 – Alkali metals Li, Na, K, Rb, Cs, Fr. Have a single electron in their outermost shell, making them very reactive. React violently in water displacing hydrogen ions to produce hydrogen gas, leaving an excess of hydroxide ions in solution in the remaining water
Group 2 – Alkaline earth metals Be, Mg, Ca, Sr, Ba, Ra. This specific group in the periodic table owes its name to their oxides that simply give basic alkaline solutions. Less reactive than alkali metals as they have two electrons in their outer shell
Group 3 – scandium and yttrium
Group 4 – titanium, zirconium and hafnium
Group 5 – vanadium, niobium and tantalum
Group 6 – chromium, molybdenum and tungsten
Group 7 – manganese, technetium and rhenium
Group 8 – iron, ruthenium and osmium
Group 9 – cobalt, rhodium and iridium
Group 10 – nickel, palladium and platinum
Group 11 – copper, silver and gold
Group 12 – zinc, cadmium and mercury
Group 13 – boron, aluminium, gallium, indium and thallium. All elements have the electron configuration s2p1 in their outer shell
Group 14 – carbon, silicon, germanium, tin and lead. All elements have the electron configuration s2p2 in their outer shell. The s- and p- orbitals may merge, forming hybrid orbitals
Group 15 – nitrogen, phosphorus, arsenic, antimony and bismuth. All elements have the electron configuration s2p3 in their outer shell. Also known as the pnictogens
Group 16 – oxygen, sulfur, selenium, tellurium and polonium. All elements have the electron configuration s2p4 in their outer shell. Also known as the chalcogens, from the Greek chalcos, meaning ‘bronze’ or just ‘metal’
Groups 13 to 16 have a trend from non-metallic to metallic down the group
Group 17 – Halogens F, Cl, Br, I, At. All elements have the electron configuration s2p5 in their outer shell. Named by Jons Jacob Berzelius after the Greek word for salt
Halogens are highly reactive because they are one electron short of a filled outer electron shell. They easily accept an electron from other atoms, and become negatively charged ions which are very stable
The group of halogens is the only periodic table group which contains elements in all three familiar states of matter at standard temperature and pressure
Group 18 – Noble gases He, Ne, Ar, Kr, Xe, Rn. Very low reactivity; their outer shell of valence electrons is full, giving them little tendency to participate in chemical reactions
All noble gases are colourless, odourless, monotonic and have very low boiling points. The atoms are spherical due to the full outer electron shell
Noble gas is translated from the German noun Edelgas, first used in 1898 by Hugo Erdmann to indicate their extremely low level of reactivity
All of the naturally occurring noble gases were discovered by William Ramsay and his assistant Morris Travers – He, Ne, Ar, Kr, Xe
Inert gas – archaic name for noble gas
Nearly every element in the periodic table can be termed either a metal or a non-metal – however a few elements with intermediate properties are referred to as metalloids or semimetals. The following elements are generally considered metalloids – B, Si, Ge, As, Sb, Te
Transition metal – any element in the d-block of the periodic table (d sub-shells are filled with electrons), including zinc, cadmium and mercury. This corresponds to groups 3 to 12 on the periodic table. Form alloys easily and are excellent conductors of electricity, e.g. copper, iron, cobalt. Valency of 2
Poor metals – the metallic elements in the p-block of the periodic table. The poor metals are distinguished from the metalloids by their significantly higher electrical conductivity values and, for elements in the same periodic table row, greater densities
Post-transition metals – the metallic elements in periods 4–6 of the periodic table, to the right of the transition elements. Since this description excludes aluminium, a period 3 metal, the post-transition elements thereby form a subset of the poor metals
Class A metals – form hard acids, e.g. iron, aluminium, titanium, sodium, calcium
Class B metals – form soft acids, e.g. lead, gold, platinum, mercury
The six platinum-group metals are ruthenium, rhodium, palladium, osmium, iridium, and platinum. They have similar physical and chemical properties, and tend to occur together in the same mineral deposits
Noble metals are metals that are resistant to corrosion and oxidation in moist air, unlike most base metals. They tend to be precious, often due to their rarity in the Earth's crust. The noble metals are considered to be ruthenium, rhodium, palladium, silver, osmium, iridium, platinum, and gold
Copper and gold are the metals whose lustre is not silver-grey. They absorb photons at the higher-energy blue end of the spectrum
Goldschmidt classification, developed by Victor Goldschmidt, is a geochemical classification which groups the chemical elements according to their preferred host phases into lithophile (rock-loving), siderophile (iron-loving), chalcophile (sulfur-loving), and atmophile (gas-loving)
Smithson Tennant is best known for his discovery of the elements iridium and osmium, which he found in the residues from the solution of platinum ores in 1803. He also contributed to the proof of the identity of diamond and charcoal
Didymium was discovered by Carl Mosander in 1841 and was so named because it is very similar to lanthanum, with which it was found. It is made up of samarium, praseodymium and neodymium. Today the term is used for a mixture of praseodymium and neodymium
GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany is a heavy ion research centre. Elements discovered at GSI: meitnerium (1982), hassium (1984), darmstadtium (1994), roentgenium (1994), bohrium (1996), and copernicium (1996)
Lending its name to a famous quarry where many rare earth minerals have been discovered, the small village of Ytterby in Sweden has been the inspiration for naming the chemical elements yttrium (Y), ytterbium (Yb), terbium (Tb) and erbium (Er)
Of the 88 natural elements, only 20 are found in the native state
Elements by atomic number
Hydrogen (H), atomic number 1 – from Greek for ‘water generator’
Metallic form of hydrogen created by Lawrence Livermore laboratory for a microsecond in 1996
Hydrogen – one proton and one electron (atomic mass = 1, atomic number = 1)
Hydrogen – only element that contains no neutrons
Pop test is a test for hydrogen. Put a lit splint into the test tube. If there is a squeaky pop, the gas will be hydrogen
Hydride – the anion of hydrogen, H−. Hydrides may refer to any compound that hydrogen forms with other elements
In 1766, Henry Cavendish was the first to recognize hydrogen gas as a discrete substance, by identifying the gas from a metal-acid reaction as ‘inflammable air’ and further finding in 1781 that the gas produces water when burned. He is usually given credit for its discovery as an element
Antihydrogen – the antimatter counterpart of hydrogen. Whereas the common hydrogen atom is composed of an electron and proton, the antihydrogen atom is made up of a positron and antiproton. First produced at CERN in 1995
Tritium (symbol T or 3H) is a radioactive isotope of hydrogen. The nucleus of tritium (sometimes called triton) contains one proton and two neutrons, whereas the nucleus of protium (the most abundant hydrogen isotope) contains no neutrons
Tritium – one proton, two neutrons and one electron (atomic mass = 3, atomic number = 1)
Deuterium, also called heavy hydrogen, is a stable isotope of hydrogen with a natural abundance in the oceans. The nucleus of deuterium, called a deuteron, contains one proton and one neutron, whereas the far more common hydrogen nucleus consists only of a proton and no neutrons
Deuterium – one proton, one neutron and one electron (atomic mass = 2, atomic number = 1)
Heavy water is dideuterium oxide, or D2O or 2H2O. It is chemically the same as normal water, H2O, but the hydrogen atoms are of the heavy isotope deuterium. Gilbert Newton Lewis isolated the first sample of pure heavy water in 1933
Helium (He), atomic number 2 – named after the Sun by Joseph Lockyer
Helium – two protons, two neutrons and two electrons (atomic mass = 4, atomic number = 2)
Along with the French scientist Pierre Janssen, Norman Lockyer is credited with discovering the gas helium
Helium has the lowest boiling point and melting point of any element
Solid helium is the coldest substance
Most helium on Earth is a result of radioactive decay. Helium is found in large amounts in minerals of uranium and thorium
An alpha particle is identical to a helium-4 nucleus [2p, 2n]
Helium is present in many natural gas deposits
USA is main producer of helium, and has a National Helium Reserve
Liquid helium is used to conduct superconducting magnets
Helium was discovered by the observation of a yellow line in the emission spectrum of the chromosphere
Helium-3 (He-3) is a light, non-radioactive isotope of helium with two protons and one neutron. It is rare on Earth, and it is sought for use in nuclear fusion research. The abundance of helium-3 is thought to be greater on the Moon
Helium-4 is a non-radioactive isotope of helium
Lithium (Li), atomic number 3 – from Greek for ‘stone’
Humphrey Davy was the first person to extract atoms of pure lithium, by electrolysis
Lithium is the lightest of Group 1 alkali metals
Lithium flame burns red
Beryllium (Be), atomic number 4 – named after beryl. Discovered by Nicholas-Louis Vauquelin in 1798. Used to make mirrors for infrared telescopes
Boron (B), atomic number 5 – from ancient Arabic and Persian names for borax
Boron is a metalloid with small atoms which only forms covalent bonds
Carbon (C), atomic number 6 – from the Latin carbo for ‘coal’ and ‘charcoal’
Carbon-12 is the more abundant of the two stable isotopes of carbon, accounting for 98.9% of carbon; it contains six protons, six neutrons and six electrons. Carbon-12 is used as the standard from which atomic masses of all nuclides are measured: its mass number is 12 by definition
Carbon-13 is the other stable isotope of carbon
Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons
Carbon is the fourth most abundant chemical element in the universe by mass after hydrogen, helium, and oxygen
Carbon does not have a standard melting or boiling point, as it sublimes (turns directly from a solid directly into a gas), at 3600oC
Amorphous carbon – free, reactive carbon that does not have any crystalline structure, e.g. coal or soot
Diamonds and graphite are allotropes of carbon
Graphite is an electrical conductor. It was named by Abraham Gottlob Werner in 1789
Carbon nanotubes (CNTs; also known as buckytubes) are allotropes of carbon with a cylindrical nanostructure. Carbon nanotubes are the strongest and stiffest materials yet discovered in terms of tensile strength and elastic modulus respectively
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and cylindrical ones are called carbon nanotubes or buckytubes
Buckminsterfullerene, also known as Buckyballs, is named after the similarity to the geodesic domes designed by Buckminster Fuller. A form of carbon with a molecule consisting of 60 carbon atoms arranged symmetrically in hexagonal and pentagonal rings to form a nearly spherical structure
Historically, graphite was called black lead or plumbago
Graphene is a one-atom-thick planar sheet of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice
Nitrogen (N), atomic number 7 – from French for ‘nitre generator’
Nitrogen is considered to have been discovered by Scottish physician Daniel Rutherford in 1772, who called it noxious air or fixed air
Nitrogen gas is very unreactive, or inert
Azide – the anion with the formula N3−
Oxygen (O), atomic number 8 – means ‘acid producer’. Named by Lavoisier
The nucleus of the most common isotope of oxygen, oxygen-16, is made of four helium-4 nuclei fused together
Liquid and solid oxygen have a bluish tinge
Oxygen is weakly magnetic
Glowing splint test is a test for oxygen. It involves a thin piece of wood (splint) being lit, with the flame blown out, leaving an ember at the tip. Upon exposure to high concentrations of oxygen, the glowing ember flares and gives a flame
Ozone (O3) is an allotrope of oxygen that is much less stable than the diatomic allotrope O2, breaking down in the lower atmosphere to normal dioxygen
Fluorine (F), atomic number 9 – from Latin fluere, meaning ‘to flow’
Fluorine is the most electronegative element and is extremely reactive
Hydrogen fluoride is the principal industrial source of fluorine
Fluoride (F-) catalyzes the diffusion of calcium and phosphate into the tooth surface, which in turn remineralizes the crystalline structures in a dental cavity
Neon (Ne), atomic number 10 – from Greek neos, meaning ‘new’
Neon is the fifth most abundant element in the universe
Sodium (Na), atomic number 11 – from natrium, the Latin name for sodium carbonate
Sodium was discovered by Humphrey Davy in 1807
Sodium – from sodanum, Roman for glasswort, plants used in glassmaking
Sodium burns with an orange-yellow flame
Magnesium (Mg), atomic number 12 – identified by Joseph Black, isolated by Humphrey Davy in 1808. Magnesium is named after Magnesia, a Prefecture of Greece
Magnesium burns with a white flame. Found in olivine and pyroxene
China produces 90% of the world’s magnesium
Aluminium (Al), atomic number 13 – name is derived from the mineral alum
Before the Hall-Heroult process was developed in the late 1880s, aluminium was very difficult to extract from its various ores. This made pure aluminium more valuable than gold. Bars of aluminium were exhibited at the Exposition Universelle of 1855
Silicon (Si), atomic number 14 – from Latin silex for ‘flint’
Over 90% of the Earth's crust is composed of silicate minerals, making silicon the second most abundant element in the Earth's crust (about 28% by mass) after oxygen
Phosphorus (P), atomic number 15 – means ‘light bearing’. The discovery of phosphorus is credited to the German alchemist Hennig Brand in 1669 while boiling urine in an attempt to extract gold. He did not publish his discovery, and phosphorus was discovered independently by Robert Boyle
Icy noctiluca – original name for white phosphorus, which is highly toxic
Red phosphorus is non-toxic and is used in match heads
Sulfur (S), atomic number 16 – from Latin word sulpur. Also known as sulphur until 1990
Sulfur has more allotropes than any other element
Sulfide – an inorganic anion of sulfur with the chemical formula S2−
Brimstone – old name for sulfur
Chlorine (Cl), atomic number 17 – from Greek chloros, meaning ‘greenish-yellow’
Chlorine condenses to form a liquid at -34oC
Chloride ion – formed when chlorine picks up one electron to form an anion
Argon (Ar), atomic number 18 – from Greek for ‘inactive’
Argon – suspected to be present in air by Henry Cavendish in 1785 but was not discovered until 1894 by Lord Rayleigh and Sir William Ramsay in an experiment in which they removed all of the oxygen and nitrogen from the air
Argon is the third most common gas in the Earth's atmosphere, at 0.93%
Argon is produced industrially by the fractional distillation of liquid air
Potassium (K), atomic number 19 – from kalium, Latin for ‘alkali’
Potassium burns with a lilac flame
Potassium was the first element that Humphrey Davy discovered, by splitting potash using electricity
Potassium is key to the transition of nerve impulses
Calcium (Ca), atomic number 20 – Latin word calcis meaning ‘lime’
Calcium burns with a red flame
Calcium is the most abundant metallic element in the body
Scandium (Sc), atomic number 21 – named after Scandinavia
Scandium is alloyed with aluminium for commercial use
Titanium (Ti), atomic number 22 – named after the Titans of Greek mythology
Titanium is a light, strong, lustrous, corrosion-resistant (including resistance to sea water and chlorine) transition metal with a white-silvery-metallic colour. It is extracted from its principal mineral ores via the Kroll process
Titanium has the highest strength-to-weight ratio of all metals
Vanadium (V), atomic number 23 – named after Freyja (also named as Vanadis) after having many previous names
Chromium (Cr), atomic number 24 – named after the Greek word chroma meaning ‘colour’, because of the many colourful compounds made from it. Discovered by Nicholas-Louis Vauquelin
Chromate ions contain chromium in an oxidation state of +6
Manganese (Mn), atomic number 25 – named after Magnesia, a Prefecture of Greece
Manganese is a metal with important industrial metal alloy uses, particularly in stainless steels
Iron (Fe), atomic number 26 – named after ferrum, Latin for ‘iron’
Iron is the most abundant element in the Earth as a whole. The majority of native iron is found at the Earth’s core. Usually alloyed to nickel
Cobalt (Co), atomic number 27 – named after the German for ‘goblin’
Swedish chemist Georg Brandt is credited with discovering cobalt
Cobalt-60 is used as a source of gamma radiation in radiotherapy
Nickel (Ni), atomic number 28 – named after the same mischievous sprite of German mythology as cobalt, albeit by an alternative name, Nickel
Nickel-62 has the highest binding energy per nucleon of any known nuclide. It is often stated that 56Fe is the ‘most stable nucleus’, but actually 56Fe has the lowest mass per nucleon (not binding energy per nucleon) of all nuclides
Russia is the largest producer of nickel
Copper (Cu), atomic number 29 – named after Cyprium, ‘metal of Cyprus’, later shortened to Cuprum
Copper burns with a blue flame
Copper has the second highest electrical conductivity of all metals, after silver
Zinc (Zn), atomic number 30 – from German zinke, meaning ‘prong’ or ‘point’
Romans used zinc but were unaware it was a metal in its own right
Zinc was first isolated by Hindus in 13th century. Largest zinc mine is in India
Zinc is used in die casting
Gallium (Ga), atomic number 31 – from Latin for Gaul
Melting point of zinc is 30oC. Used in transistors and LEDs
Germanium (Ge), atomic number 32 – named after Germania, the Latin name for Germany
Germanium is a lustrous, hard, greyish-white metalloid
Germanium is a semiconductor used in electronics
Arsenic (As), atomic number 33 – named after the Persian word for ‘gold-coloured’
Arsenic smells of garlic when heated
Marsh test – method for detection of arsenic
Selenium (Se), atomic number 34 – named after the Greek goddess of the moon, Selene
Selenium exists in two allotropes – a red non-metal and a dull grey semiconducting metalloid
The chief commercial uses for selenium today are in glassmaking and in pigments. Selenium is a semiconductor and is used in photocells
Bromine (Br), atomic number 35 – named after the Greek bromos, meaning ‘stench’
Bromine is extracted from seawater (brine)
Bromine is liquid at room temperature
Bromide – bromine ion (Br-)
Krypton (Kr), atomic number 36 – named after the Greek for ‘the hidden one’
The first krypton compound, krypton difluoride, was produced in 1963
Krypton fluoride laser is used in the production of semiconductor integrated circuits
Rubidium (Rb), atomic number 37 – named after the Latin rubidus, meaning ‘dark red’
Named by Bunsen because of the two deep red lines in its spectrum
Strontium (Sr), atomic number 38 – named after the Scottish village of Strontian, having been discovered in the lead mines there
Strontium itself was discovered in 1798 by Thomas Charles Hope, and metallic strontium was first isolated by Sir Humphry Davy in 1808 using electrolysis
Strontium is used in flares as it gives off a bright light
Strontium burns with a red flame
Strontium-90 produced by nuclear weapon tests
Strontium-90 replaces calcium in bones and bone marrow
Yttrium (Y), atomic number 39 – named after the Swedish village of Ytterby
Zirconium (Zr), atomic number 40 – named after the mineral zircon
Zirconium has similar properties and applications to titanium. Discovered by Martin Klaproth
Niobium (Nb), atomic number 41 – named after Niobe, the daughter of Tantalus in Greek mythology
Niobium was known as columbium
At low temperatures, niobium is a superconductor
Niobium is used in the manufacture of the nozzles of jet and rocket engines
Molybdenum (Mb), atomic number 42 – named after the ancient Greek word for ‘lead’
Molybdenum is an important biological catalyst, notably in nitrogenase
Technetium (Tc), atomic number 43 – from the Greek tekhnitos meaning ‘artificial’
Technetium is the element with the lowest atomic number element without any stable isotopes
In 1937, technetium became the first predominantly artificial element to be produced, hence its name
Technetium-99m is a metastable nuclear isomer of technetium-99 that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medical radioisotope. Technetium-99m when used as a radioactive tracer can be detected in the body by medical equipment (gamma cameras)
Ruthenium (Ru), atomic number 44 – named after Ruthenia, the Latin name for Russia
Ruthenium usually occurs as a minor component of platinum ores
Rhodium (Rh), atomic number 45 – named after the Greek rhodon meaning ‘rose’
William Hyde Wollaston named the element, after he extracted the pure metal from a rose-coloured solution he had made from a platinum ore
Rhodium is used in catalytic converters and optical reflectors
Palladium (Pd), atomic number 46 – named after the asteroid Pallas
Palladium was discovered in platinum ores in 1803 by William Hyde Wollaston, who named it after the asteroid Pallas, which was named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas
Palladium is a rare silver white transition metal of the platinum group, resembling platinum chemically
The largest use of palladium is in catalytic converters
Palladium forms a versatile catalyst and speeds up hydrogenation and dehydrogenation reactions
Silver (Ag), atomic number 47 – named after its Latin name, argentum
Silver is a soft, white, lustrous transition metal, and has the highest electrical conductivity of any element and the highest thermal conductivity of any metal
Cadmium (Cd), atomic number 48 – named after the Greek word for the mineral calamine
Cadmium occurs as a minor component in most zinc ores and therefore is a byproduct of zinc production
Paint containing cadmium pigments was used by the Impressionists
Indium (In), atomic number 49 – named after indigo
Indium was discovered in 1863 and named for the indigo blue line in its spectrum that was the first indication of its existence in zinc ores
Tin (Sn), atomic number 50 – named after the Latin word ‘stannum’
Alpha tin and beta tin – allotropes of tin
Tin has greatest number of stable isotopes (10) of all elements
Antimony (Sb), atomic number 51 – named after the Latin word ‘stibium’
Antimony is a silvery lustrous grey metalloid, it is found in nature mainly as antimony sulfide, commonly known as stibnite. Very similar properties to arsenic
Tellurium (Te), atomic number 52 – named after the Latin word for ‘earth’ (tellus) by Martin Klaproth
Tellerium is a brittle, mildly toxic, silver-white metalloid. Garlic smell. The only element that can bind chemically to gold
Tellurium-128 is the isotope with the longest half life among all radionuclides
Iodine (I), atomic number 53 – named after the Greek iodes meaning ‘violet’ or ‘purple’
Iodine was discovered in seaweed by French chemist Bernard Courtois in 1811
In its elemental form, iodine exists as a solid composed of diatomic molecules. It has a deep blue-black colour. It sublimes at room temperature, and forms a thick brownish liquid at 114oC. Iodine test is used to test for the presence of starch
Iodide – iodine ion (I-)
Xenon (Xe), atomic number 54 – named after the Greek xenos meaning ‘stranger’
Xenon is used in light bulbs, lasers, and arc lamps for cinema projection
In 1962 Neil Bartlett produced the first known compound of a noble gas, xenon hexafluoroplatinate
Caesium (Cs), atomic number 55 – named after the Latin caesius, ‘sky blue’, due to the bright blue lines in its emission spectrum
Caesium is the least electronegative element having a stable isotope, caesium-133
Caesium has a melting point of 28oC
Caesium is used in atomic clocks and the definition of a second
Barium (Ba), atomic number 56 – named after the Greek word for ‘heavy’
In 1938, Otto Hahn and Fritz Strassmann detected the element barium after bombarding uranium with neutrons; simultaneously, they communicated these results to Lise Meitner. Meitner, and her nephew Otto Robert Frisch, correctly interpreted these results as being nuclear fission. Meitner realized that fission converted mass into energy
Barium is used to give green colours in fireworks
Barium meal – barium sulfate. Barium ions are opaque to X-rays
Lanthanum (La), atomic number 57 – named after the Greek for ‘to lie hidden’, as it was an impurity in cerite
Lanthanum is the first element of the lanthanide series
Cerium (Ce), atomic number 58 – named after the dwarf planet Ceres by Berzelius
Cerium was the first lanthanide to be discovered, and is the most abundant
Cerium was discovered in Sweden by Jons Jacob Berzelius, and independently in Germany by Martin Klaproth both in 1803
Praseodymium (Pr), atomic number 59 – named after the Greek for ‘green-coloured twin’
Neodymium (Nd), atomic number 60 – named after the Greek for ‘coloured twin’. Used to make magnets
Promethium (Pm), atomic number 61 – named after Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans
All of the isotopes of Promethium are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium
Samarium (Sm), atomic number 62 – named after the mineral samarskite from which it was isolated. The mineral itself was earlier named after a Russian mine official, Colonel Vasili Samarsky-Bykhovets, who thereby became the first person to have a chemical element named after him
Europium (Eu), atomic number 63 – named after Europe
Most applications of europium exploit the phosphorescence of europium compounds
Gadolinium (Gd), atomic number 64 – named after the chemist Johan Gadolin. Used in comtrol rods in nuclear reactors
Terbium (Tb), atomic number 65 – named after the Swedish village of Ytterby
Dysprosium (Dy), atomic number 66 – named aftyer the Greek dysprositos, meaning ‘hard to obtain’
Holmium (Ho), atomic number 67 – named after Holmia, the Latin name for Stockholm
Erbium (Er), atomic number 68 – named after the Swedish village of Ytterby
Thulium (Tm), atomic number 69 – named after Thule, a mysterious northerly part of Scandanavia
Ytterbium (Yb), atomic number 70 – named after the Swedish village of Ytterby
Lutetium (Lu), atomic number 71 – named after Lutetia, the Latin name for Paris
Hafnium (Hf), atomic number 72 – named after Hafnia, the Latin name for Copenhagen
Hafnium is a good material for neutron absorption in control rods in nuclear power plants
Hafnium oxide enables the size of each transistor on a microprocessor chip to be reduced
Tantalum (Ta), atomic number 73 – named after Tantalus, from Greek mythology. The name was chosen because tantalum’s oxide would not react with acids
Tungsten (W), atomic number 74 – named after wolfram, from the German for ‘wolf spittle’
Tungsten – from Nordic words for ‘heavy stone’
Tungsten has the highest melting point
Tungsten is used to make the filaments of incandescent lamps
Tungsten carbide (chemical formula WC) is used in industrial machinery, cutting tools, abrasives, armour-piercing rounds, other tools and instruments, and jewellery
Rhenium (Re), atomic number 75 – named after the River Rhine
Rhenium has the highest boiling point
Rhenium was the last element to be discovered that has at least one stable isotope
Osmium (Os), atomic number 76 – named after the Greek osme meaning ‘smell’
Osmium is a hard, brittle, blue-gray or blue-black transition metal in the platinum family and is the densest naturally occurring element
Its alloys with platinum, iridium, and other platinum group metals are employed in fountain pen nibs and electrical contacts
Iridium (Ir), atomic number 77 – named after the Greek goddess Iris
Iridium is the second-densest element and is the most corrosion-resistant metal
The principal use of iridium is as a hardening agent in platinum alloys
Platinum (Pt), atomic number 78 – named after plata, the Spanish word for ‘silver’. Conquistadors named the metal platino del Pinto – the ‘little silver’ of the Rio Pinto
Platinum was discarded as waste when first discovered by Antonio de Ulloa in Ecuador
Platinum is alloyed with iridium to increase hardness
Platinum is the least reactive of all metals
Gold (Au), atomic number 79 – named after aurum, the Latin word for gold
The name comes from Indo-european word ghel, meaning ‘yellow’
Gold is thought to have been produced in supernova nucleosynthesis to seed the dust from which the Solar System formed
Mercury (Hg), atomic number 80 –named after hydrargyrum, meaning watery or liquid silver
The element was named after the Roman god Mercury
Mercury is also called quicksilver
A heavy, silvery transition metal, mercury is the only metal that is liquid at or near room temperature and pressure
Mercury melts at -39 degrees C
Mercury has the lowest conductivity of all metallic elements
Thallium (Tl), atomic number 81 – named after the Greek thallos, meaning ‘a green shoot or twig’
This soft gray malleable poor metal resembles tin but discolours when exposed to air. Discovered by William Crookes. Named after the bright green light observed in its spectrum
Lead (Pb), atomic number 82 – named after the Latin word for lead, plumbum
Lead burns with a blue flame
Lead-208 is the heaviest stable isotope
Lead (II, IV) oxide, also called red lead is used in the manufacture of batteries, lead glass and rust-proof primer paints
Small-scale lead smelting began 9000 years ago in Turkey and Iran
Bismuth (Bi), atomic number 83 – named after (possibly) the Arabic bi ismid, meaning ‘having the properties of antimony’ or German words weisse masse or wismuth (‘white mass’), translated in the mid sixteenth century to New Latin bisemutum
Bismuth is generally considered to be the last naturally occurring stable, non-radioactive element on the periodic table
Bismuth-209 was long thought to have the heaviest stable nucleus of any element, but in 2003, Noel Coron discovered that 209Bi undergoes alpha decay with a half-life of approximately 1.9×1019 years
Polonium (Po), atomic number 84 – named after Poland. Discovered in pitchblende by Marie and Pierre Curie
Polonium atoms are created inside uranium ores
Polonium is sometimes considered a true metal, sometimes a metalloid
Polonium has 33 known isotopes, all of which are radioactive
Astatine (At), atomic number 85 – named after the Greek astatos, meaning ‘unstable’
Astatine occurs on Earth only as the result of the radioactive decay of certain heavier elements. Longest lived isotope has a half-life of eight hours
Radon (Rn), atomic number 86 – named after Radium Emanation by Friedrich Ernst Dorn, who discovered the element in 1900
Radon is formed as part of the normal radioactive decay chain of uranium or thorium. Radon is often the single largest contributor to an individual's background radiation dose
Radon is also the only gas under normal conditions that only has radioactive isotopes
Francium (Fr), atomic number 87 – named after France
Francium has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element (after astatine). Francium is a highly radioactive metal. Francium was discovered by Marguerite Perey in France
Francium usually occurs as a product of the decay of actinium
Radium (Ra) – atomic number 88 – named after the Latin radius, meaning ‘ray’
Radium has a silvery metallic appearance
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, and clocks. Radium is treated as calcium by the body, and deposited in the bones, where radioactivity degrades marrow and can mutate bone cells
Actinium (Ac), atomic number 89 – named after the Greek actinos, meaning ‘ray’
A soft, silvery-white radioactive metal, actinium gives its name to the actinide series
Thorium (Th), atomic number 90 – named after the Norse god of thunder. Discovered by Jons Jacob Berzelius
A thorium fuel cycle offers several potential advantages over a uranium fuel cycle – including much greater abundance on Earth, superior physical and nuclear fuel properties, and reduced nuclear waste production. However, it suffers from higher production and processing costs, and lacks significant weaponization potential. Since 2008, nuclear energy experts have become more interested in thorium to supply nuclear fuel in place of uranium to generate nuclear power
The most stable isotope, thorium-227, has a half-life of 14 billion years
Protactinium (Pa), atomic number 91 – named after the Greek protos, meaning ‘before’. The element transmutes into actinium when it decays
Uranium (Ur), atomic number 92 – named after the Greek god Uranus by Martin Klaproth
Yellowcake – a kind of uranium concentrate powder, an intermediate step in the processing of uranium ores
Three isotopes of uranium exist in nature – U-234, U-235 and U-238. U-235 is fissile
Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Uranium enrichment takes place in a gas cantrifuge
Neptunium (Np), atomic number 93 – named after the planet Neptune
Neptunium was the first 'heavier than uranium' element to be produced artificially
Neptunium was discovered by Edwin McMillan and Philip Abelson in 1940 at the Berkeley Radiation Laboratory. The team produced the neptunium isotope 239Np by bombarding uranium with slow moving neutrons. It was the first transuranium element produced synthetically
Plutonium (Pu), atomic number 94 – named after the planet Pluto
The first nuclear test, ‘Trinity’, and the atomic bomb dropped on Nagasaki, both had cores of plutonium-239
Plutonium was first produced at Berkeley in 1940 by a team headed by Glenn Seaborg
Plutonium is the heaviest primordial element by virtue of its most stable isotope, plutonium-244
Americium (Am), atomic number 95 – named after the Americas. Americium was first produced in 1944 by bombarding plutonium with neutrons in a cyclotron at Berkeley
Curium (Cm), atomic number 96 – named after Marie Curie. Produced before Americium
Berkelium (Bk), atomic number 97 – named after the city of Berkeley, California, the location of the University of California Radiation Laboratory where it was discovered in 1949
Californium (Cf), atomic number 98 – named after California, and first made in 1950 at the University of California Radiation Laboratory in Berkeley
Einsteinium (Es), atomic number 99 – named after Albert Einstein. First detected in the fallout of the first hydrogen bomb test, in 1952
Fermium (Fm), atomic number 100 – named after Enrico Fermi. First detected in the fallout of the first hydrogen bomb test, in 1952
Mendelevium, atomic number 101 – named after Dmitri Mendeleev. Produced in 1955 by firing alpha particles at einsteinium
Elements 102-106 were discovered at the Berkeley Heavy Ion Linear Accelerator (HILAC) by Glenn Seaborg and Albert Ghiorso
Nobelium (No), atomic number 102 – named after Alfred Nobel
Lawrencium (Lr), atomic number 103 – named after Ernest Lawrence, the inventor of the cyclotron
Rutherfordium (Rf), atomic number 104 – named after Ernest Rutherford
Dubnium (Db), atomic number 105 – named after Dubna, a town in Russia home to the Joint Institute for Nuclear Research
Seaborgium (Sg), atomic number 106 – named after Glenn Seaborg. The IUPAC adopted unnilhexium (symbol Unh) as a temporary, systematic element name. Seaborgium is the only element to have been named by someone who was alive at the time
Bohrium (Bh), atomic number 107 – named after Niels Bohr. Bohrium was first convincingly synthesized in 1981 by a German research team led by Peter Armbruster at the Institute for Heavy Ion Research in Darmstadt
Hassium (Hs), atomic number 108 – derived from the Latin name for the German state of Hesse, where the Darmstadt is located
Meitnerium (Mt), atomic number 109 – named after Lise Meitner, a co-discoverer of protactinium (with Otto Hahn) and one of the discoverers of nuclear fission
Darmstadtium (Ds), atomic number110 – named after Darmstadt, where the element was discovered
Roentgenium (Rg), atomic number 111 – named after Wilhelm Rontgen. Accepted as a permanent name in 2004. Previously the element was known under the temporary IUPAC systematic element name unununium (Uuu)
Copernicium (Cn), atomic number 112 – named after Nicolaus Copernicus. Accepted as a permanent name in 2010. Previously the element was known under the temporary IUPAC systematic element name Ununbium (Uub). Copernicium is made from fusing of zinc and lead. Copernicium has been observed as decay products of flerovium
Nihonium (Nh), atomic number 113 – named after one of the two Japanese pronunciations for the name of Japan. It is the first element to be named after a location in East Asia. Previously the element was known under the temporary IUPAC systematic element name Ununtrium (Uut)
Flerovium (Fl), atomic number 114 – named after Russian physicist Georgy Flyorov, the founder of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was discovered. Accepted as a permanent name in 2012. Previously the element was known under the temporary IUPAC systematic element name Ununquadium (Uuq)
Moscovium (Mc), atomic number 115 – named after the Moscow Oblast which is the location of the Joint Institute for Nuclear Research. Previously the element was known under the temporary IUPAC systematic element name Ununpentium (Uup)
Livermorium (Lv), atomic number 116 – named after the Lawrence Livermore National Laboratory, which was involved in its discovery in 2000. Accepted as a permanent name in 2012. Previously the element was known under the temporary IUPAC systematic element name Ununhexium (Uuh)
Tennessine (Ts), atomic number 117 – named after the state of Tennessee which is home to Oak Ridge National Laboratory. Synthesized in 2010. Previously the element was known under the temporary IUPAC systematic element name Ununseptium (Uus)
Oganesson (Og), atomic number 118 – named after the Russian nuclear physicist Yuri Oganessian who led the team that synthesized it. Previously the element was known under the temporary IUPAC systematic element name Ununoctium (Uuo)
Ununennium, also known as eka-francium or element 119, is the temporary name of a chemical element that has the temporary symbol Uue. To date, attempted syntheses of this element have been unsuccessful
Unbihexium – an undiscovered superheavy chemical element with temporary symbol Ubh and atomic number 126. The element is predicted to be near the centre of the hypothesized island of stability, and thus may have a very long half-life
Acids
Acid – any chemical compound that, when dissolved in water, gives a solution with a hydrogen ion activity greater than in pure water, i.e. a pH less than 7.0
Acid – from the Latin acidus, meaning ‘sour’
There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition
Aqua Regis – mixture of hydrochloric and nitric acids, capable of dissolving gold and platinum
Carboxylic acids – organic acids characterized by the presence of at least one carboxyl group (COOH), including formic acid, acetic acid, stearic acid and benzoic acid
Formic acid – methaonic acid. In nature, it is found in the stings and bites of many insects of the order Hymenoptera, mainly ants and is also present in stinging nettles
Ethanoic acid – acetic acid
Stearic acid is mainly used in the production of detergents, soaps, and cosmetics such as shampoos and shaving cream products
Benzoic acid – C7H6O2.A colourless crystalline solid and the simplest aromatic carboxylic acid
Dicarboxylic acid – an organic compound containing two carboxyl functional groups , including malice acid and oxalic acid
Malic acid – a dicarboxylic acid which is made by all living organisms, contributes to the pleasantly sour taste of fruits, and is used as a food additive
Oxalic acid is found in rhubarb leaves
Acetylsalicylic acid – aspirin
Carbonic acid – H2CO3. It is also a name sometimes given to solutions of carbon dioxide in water
Hydrochloric acid – HCl. Formerly known as muriatic acid
Hydrofluoric acid – HF. A solution of hydrogen fluoride in water. It is a valued source of fluorine and used to manufacture PTFE. Hydrofluoric acid is a highly corrosive acid, capable of dissolving many materials, including glass
Hydrogen cyanide – HCN. Also known as prussic acid, can be obtained from almonds
Nitric acid – HNO3. Also known as aqua fortis
Nitrous acid – HNO2
Phenol, also known under an older name of carbolic acid, is a colourless crystalline solid with a typical sweet tarry odour. Its chemical formula is C6H5OH and its structure is that of a hydroxyl group (-OH) bonded to a phenyl ring; it is thus an aromatic compound. Phenol was first extracted from coal tar, but today is produced on a large scale from petroleum
Phosphoric acid – H3PO4. A mineral (inorganic) acid
Salicylic acid – chemically similar but not identical to the active component of aspirin. Extracted from the bark of the willow tree
Sulfuric acid – H2SO4. Also known as oil of vitriol. Used to etch glass
Tartaric acid – a white crystalline organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds, and is one of the main acids found in wine. It is added to other foods to give a sour taste, and is used as an antioxidant
Uric acid – C5H4N4O3. High blood concentrations of uric acid can lead to gout
Strong acids and bases completely disassociate in water
Hydrogen halides – gases that dissolve in water to give acids
When a metal is added to an acid, hydrogen gas is one of the reaction products
Salts
Salt – a solid compound formed from the combination of an acid and a base by the replacement of hydrogen ions in the acid by positive ions in the base
There are several varieties of salts. Salts that hydrolyze to produce hydroxide ions when dissolved in water are basic salts, whilst those that hydrolyze to produce hydronium ions in water are acidic salts. Neutral salts are those that are neither acid nor basic salts
The name of a salt starts with the name of the cation (e.g., sodium or ammonium) followed by the name of the anion (e.g., chloride or acetate)
Common salt-forming anions include: carbonate (CO3), nitrate (NO3), nitrite (NO2), phosphate (PO4), sulfate (SO4)
Gypsum – hydrous calcium sulfate
Glauber’s salt – hydrated sodium sulfate
Green vitriol – ferrous (iron) sulfate
White vitriol – zinc sulfate
Iron sulfate or ferrous sulfate is the chemical compound with the formula FeSO4, known since ancient times as copperas
Copper sulfate (CuSO4) – used as a herbicide, fungicide and pesticide. Blue vitriol
Alum – a white crystalline double sulfate of aluminum
Epsom salts – hydrated magnesium sulphate
Potash – any of various mined and manufactured salts that contain potassium in water-soluble form
Potassium chloride is used as a substitute for table salt by those seeking to reduce sodium intake so as to control hypertension. A metal halide salt. Potassium chloride was historically known as ‘muriate of potash. Used for making fertilizer
Potassium nitrate – KNO3. Saltpetre. A naturally occurring mineral source of nitrogen that constitutes a critical oxidising component of black powder gunpowder
Sodium nitrate – NaNO3 is also known as Chile saltpetre
Hydrochlorides are salts resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (mostly amines)
Potassium bromide – KBr. Widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries
Hydrocarbons
Hydrocarbon – an organic compound consisting entirely of hydrogen and carbon
Alkane – acyclic saturated hydrocarbon (a long chain of carbon linked together by single bonds) formula CnH2n+2. Alkanes:
Methane – CH4
Ethane – C2H6
Propane – C3H8
Butane – C4H10
Octane – C8H18
The trivial (non-systematic) name for alkanes is paraffins
Alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond. The simplest alkenes, with only one double bond and no other functional groups, form a homologous series of hydrocarbons with the general formula CnH2n. The simplest alkene is ethylene (C2H4)
Propene – also known as propylene, is an unsaturated organic compound having the chemical formula C3H6
Alkyne – an unsaturated hydrocarbon containing a triple carbon-carbon bond, formula CnH2n-2. The simplest alkyne is acetylene (C2H2)
Benzene – C6H6. August Kekule said that he had discovered the ring shape of the benzene molecule after having a day-dream of a snake seizing its own tail
Toluene – methylbenzene. Widely used as an industrial feedstock and as a solvent
Methyl group – an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms -CH3. The group is often abbreviated Me
An alkyl group, generally abbreviated with the symbol R, is a functional group or side-chain that, like an alkane, consists solely of single-bonded carbon and hydrogen atoms, for example a methyl or ethyl group
Naphthalene – a white crystalline strong-smelling hydrocarbon made from coal tar or petroleum and used in organic synthesis and as a fumigant in mothballs
Compounds
Empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of hydrogen peroxide, or H2O2, would simply be HO
Molecular formulas indicate the simple numbers of each type of atom in a molecule of a molecular substance, e.g. hydrogen peroxide is H2O2
Structural formula of a chemical compound is a graphic representation of the molecular structure, showing how the atoms are arranged
Ammonia – NH3. Also known as azane
Aniline – the first industrial-scale use of aniline was in the manufacture of mauveine, a purple dye discovered in 1856 by William Henry Perkin, while he was attempting to synthesize quinine
Bicarbonate – an intermediate form in the deprotonation of carbonic acid. It is an anion with the chemical formula HCO3−
Bicarbonate of soda (NaHCO3) is also known as sodium bicarbonate and baking soda
Calamine – either a mixture of zinc oxide (ZnO) with about 0.5% ferric oxide (Fe2O3) or a zinc carbonate compound. It is the main ingredient in calamine lotion
Calcium carbide – CaC2. Its main use industrially is in the production of acetylene
Calcium hydroxide, also known as slaked lime, is a chemical compound with the chemical formula Ca(OH)2. It is a colourless crystal or white powder, and is obtained when calcium oxide (called lime or quicklime) is slaked with water
Fixed air – old name for carbon dioxide, discovered by Joseph Black. First gas to be isolated
Chlorate – the inorganic group ClO3 or a compound containing it
Chloroform – CHCl3, Trichloromethane, first used as an anesthetic in 1847 by James Simpson
Copper carbonate – CuCO3. A blue-green compound forming part of the verdigris patina that is found on weathered brass, bronze, and copper
Cyanide – a carbon atom triple-bonded to a nitrogen atom. Smells of almonds
Ethyl acetate is used in glues and nail polish removers
Formaldehyde – H2CO. Also known as methanal. The simplest aldehyde, it was first synthesized by the Russian chemist Aleksandr Butlerov but was conclusively identified by August Wilhelm von Hofmann. Used as a preservative
Gallium arsenide – GaAs. A semiconductor used in the manufacture of integrated circuits
Hydrogen cyanidc – HCN. Also known as prussic acid. First isolated from Prussian blue pigment
Hydrogen peroxide – H2O2
Hydroxyl – a compound containing an oxygen atom bound covalently with a hydrogen atom. The hydroxyl anion (OH−) is called hydroxide
Hydrazine – N2H4. Mainly used as a foaming agent in preparing polymer foams. It is used in various rocket fuels and to prepare the gas precursors used in air bags
Rust is an iron oxide
Ochre – of a moderate orange-yellow colour; any of various earths containing silica and alumina and ferric oxide. Hydrated iron oxide
Iron sulfide – FeS
Iron pyrites – FeS2. Known as fools’ gold
Limewater – saturated solution of calcium hydroxide, Ca(OH)2
Limewater is used to test for presence of carbon dioxide. When carbon dioxide passes through it turns from a colourless to a cloudy white substance (calcium carbonate)
Luminol exhibits chemiluminescence, with a striking blue glow, when mixed with an appropriate oxidizing agent. Luminol is used by forensic investigators to detect trace amounts of blood left at crime scenes, as it reacts with iron found in haemoglobin
Magnesium hydroxide – Mg(OH)2. Also known as milk of magnesia
Methanol – CH3OH. Also known as ‘wood alcohol’ because it was once produced chiefly as a byproduct of the destructive distillation of wood. Methanol burns in oxygen (including open air), forming carbon dioxide and water
Morphine – was first isolated in 1804 by Friedrich Serturner, and first commercially sold by Merck in 1827
Nitrocellulose – when used as a propellant or low-order explosive, it is known as guncotton. Properties discovered by Christian Schonbein in 1846. Also known as collodion
Nitroglycerin – a heavy, colourless, oily, explosive liquid obtained by nitrating glycerol. It is used in the manufacture of explosives, specifically dynamite. It was discovered by chemist Ascanio Sobrero in 1847
Nitrous oxide – N2O
Permanganate – the general name for a chemical compound containing the manganate (VII) ion (MnO4−). Because manganese is in the +7 oxidation state, the manganate (VII) ion is a strong oxidizing agent
Peroxide – a compound with an oxygen-oxygen single bond of the peroxide anion
Phosgene – COCl2. Colourless gas used as a chemical weapon
Polychlorinated biphenyls (PCB) were widely used as dielectric and coolant fluids. Due to PCBs' environmental toxicity and classification as a persistent organic pollutant, PCB production was banned in 2001
Potassium bitartrate – KC4H5O6. A byproduct of winemaking. In cooking it is known as cream of tartar
Potassium cyanide – KCN. A colourless crystalline compound, similar in appearance to sugar, that is highly soluble in water
Potassium permanganate – KMnO4. Crystals are a purple colour
Red lead – Pb3O4. Used in the manufacture of batteries, lead glass and rust-proof paint
Silica – silicon dioxide SiO2. Main component of quartz
Silica gel absorbs water from the air
Silicon carbide – SiC. A ceramic compound that is manufactured on a large scale for use mainly as an abrasive (where it is often known by the trademark carborundum) but also occurs in nature as the extremely rare mineral moissanite
Smelling salts, also known as spirit of hartshorn or sal volatile, are chemical compounds used for arousing consciousness. The usual active compound is ammonium carbonate, a colorless-to-white, crystalline solid
Sodium carbonate – Na2CO3. Also known as washing soda, soda ash and soda crystals
Sodium chlorate NaClO3. Decomposes above 250 °C to release oxygen and leave sodium chloride
Sodium hypochlorite – NaClO. When dissolved in water it is commonly known as bleach
Sodium hydroxide – NaOH. Also known as Caustic soda or lye
syn-Propanethial-S-oxide is a gas that acts as a lachrymatory agent (triggers tearing and stinging on contact with the eyes). The chemical is released from onions as they are sliced
Titanium dioxide – TiO2. Also known as titania, has a wide range of applications, from paint to sunscreen to food colouring. Used in self-cleaning windows
Thiols are used as odorants to assist in the detection of natural gas, as they smell of garlic. Thiols are often referred to as mercaptans
Friedrich Wohler obtained urea by treating silver isocyanate with ammonium chloride. This was the first time an organic compound was artificially synthesized from inorganic starting materials
Guy-Lussac and Humboldt discovered that water is formed by two parts of hydrogen and one part of oxygen in 1805. Acidic water has more H+ ions (protons), less OH- ions
Water can disassociate to form equal numbers of positively charged hydrogen ions (H+) and negatively charged hydroxyl ions (OH-). Substances that disturb this balance when added to water are called acids and alkalis
Alloys
Alloy – a mixture of metals, or a mixture of a metal with a non-metal in which the metal is the major component
Electrum is a naturally occurring alloy of gold and silver, with trace amounts of copper and other metals
Bronze – alloy of copper and tin
Brass – alloy of copper and zinc
Yellow metal – also known as muntz metal, is a form of brass with about 60% copper, 40% zinc and a trace of iron
Bell metal – form of bronze used to make bells, usually 78% copper and 22% tin
Dutch metal is a form of brass being an alloy of copper, 84% and zinc 16%
Gunmetal – alloy of copper, tin and zinc
Pinchbeck – alloy of copper and zinc
Pewter – a malleable metal alloy, traditionally 85 – 99% tin, with the remainder consisting of copper, antimony, bismuth and lead
Soft solder – alloy of lead and tin
White gold – an alloy of gold and at least one white metal, usually nickel, manganese or palladium
Sterling silver is an alloy of silver containing 92.5% pure silver and 7.5% other metals, usually copper. The minimum millesimal fineness is 925
Britannia silver is an alloy of silver containing 95.84% silver, with the balance usually copper
Fine silver is at least 99.9% pure
Sheffield plate – copper, silver-plated by fusion
Stainless steel – iron, carbon and chromium, with a minimum of 10.5% chromium content by mass
Steel – an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade
Mild steel contains less than 0.25% carbon
Medium-carbon steel contains 0.3 – 0.7% carbon
High-carbon steel contains 0.8 – 2% carbon. Strengthened by heat treatment
Cast iron (carbon > 2%) tends to be brittle
Wrought iron is an iron alloy with a very low carbon content. Wrought iron is tough, malleable, ductile and easily welded. Purest form of iron, relatively free from carbon and other impurities. Has fibrous inclusions, known as slag
Britannia metal – a pewter-type alloy favoured for its silvery appearance and smooth surface. The composition is approximately 93% tin, 5% antimony, and 2% copper
Nickel silver is a copper alloy with nickel and often zinc
Electro-plated nickel silver (EPNS) is used in zippers, keys, and costume jewellery
Alnico – a family of iron alloys which in addition to iron are composed primarily of aluminium (Al), nickel (Ni) and cobalt (Co). They also include copper, and sometimes titanium. Alnico alloys are ferromagnetic and are used to make permanent magnets
Cupronickel is used in many silver-coloured modern circulation coins
Polymers
Polymer – a large molecule made up of chains or rings of linked monomer units
Polyvinyl chloride (PVC) is the third-most widely produced plastic, after polyethylene and polypropylene
Rayon is a manufactured regenerated cellulosic fibre. Rayon is produced from naturally occurring polymers and therefore it is not a truly synthetic fibre, nor is it a natural fibre
Viscose is a viscous organic liquid used to make rayon and cellophane. Cellulose from wood or cotton fibres is treated with sodium hydroxide, and then mixed with carbon disulfide to form cellulose xanthate, which is dissolved in more sodium hydroxide. The resulting viscose is extruded into an acid bath either through a slit to make cellophane, or through a spinneret to make viscose rayon
Elastomer – a polymer that can be stretched and returns to its original shape
Neoprene – a family of synthetic rubbers that are produced by polymerization of chloroprene
Carbon fibre composite – a polymer which contains carbon fibres
A cross-link is a bond that links one polymer chain to another. Vulcanization is an example of cross-linking
Nylon is a generic designation for a family of synthetic polymers known generically as aliphatic polyamides
While most polyurethanes are thermosetting polymers that do not melt when heated, thermoplastic polyurethanes are also available
Polyethylene (polythene) is a thermoplastic polymer consisting of long chains of the monomer ethylene (ethene)
Bakelite is formed from an elimination reaction of phenol with formaldehyde
Acetate – a type of rayon
Crystallography
Crystal – a solid material, whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions
Lattice – a geometric arrangement of the points in space at which the atoms, molecules, or ions of a crystal occur
The trigonal crystal system is often confused with the rhombohedral lattice system
The seven lattice systems are a grouping of crystal structures according to the axial system used to describe their lattice – triclinic, monoclinic, orthorhombic, rhombohedral, tetragonal, hexagonal and cubic
Crystal habit – the measured, tangible external shape of individual crystals or groups thereof
Bragg’s Law gives the angles for coherent and incoherent scattering from a crystal lattice
Dimorphism – the property of certain substances that enables them to exist in two distinct crystalline forms
Ionic crystal – a crystal consisting of ions bound together by their electrostatic attraction, e.g. sodium chloride
Polymorphism – the ability of a solid material to exist in more than one form or crystal structure, e.g. the minerals calcite and aragonite, both forms of calcium carbonate
X-ray crystallography – the distance between adjacent planes in the regular matrix within crystals is similar to the X-ray wavelength so X-rays can discern the relative position of things within crystals
Processes
Bayer process – refines bauxite to produce alumina (aluminium oxide). The aluminium oxide must be purified before it can be refined to aluminium metal
Claus process – the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide
Contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. Vanadium oxide is the catalyst employed. Process was patented in 1831 by British vinegar merchant Peregrine Phillips
Frasch process – a method to extract sulfur from underground deposits
Fischer-Tropsch process – a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen into liquid hydrocarbons
Haber process (also called the Haber-Bosch process) produces ammonia from methane gas and molecular nitrogen . The ammonia from the Haber process is then converted into nitric acid (HNO3) in the Ostwald process
Hall-Heroult process is the major industrial process for smelting aluminium
Kroll process produces metallic titanium
Leblanc process was the industrial process for the production of soda ash (sodium carbonate) used throughout the 19th century, named after its inventor, Nicolas Leblanc. The process gradually became obsolete after the development of the Solvay process
Solvay process, also referred to as the ammonia-soda process, is the major industrial process for the production of soda ash (sodium carbonate)
Wacker process or the Hoechst-Wacker process originally referred to the oxidation of ethylene to acetaldehyde by oxygen in water in the presence of a palladium tetrachloride catalyst. The same basic reaction is currently used to produce aldehydes and ketones from a number of alkenes with the Monsanto process for producing acetic acid
Glossary
Absorption – the incorporation of a substance in one state into another of a different state (e.g., liquids being absorbed by a solid or gases being absorbed by a liquid)
Adsorption – the physical adherence or bonding of ions and molecules onto the surface of another phase (e.g., reagents adsorbed to solid catalyst surface)
Aerosol – a gaseous suspension of fine solid or liquid particles
Affinity – a force that binds the atoms of a molecule together
Alcohol – an organic compound in which the hydroxyl functional group (-OH) is bound to a carbon atom
Aldehyde – an organic compound containing a formyl group. Many fragrances are aldehydes
Aliphatic compound – has no delocalized electrons. Atoms bonded by covalent bonds. Does not contain aromatic rings. Aliphatics include alkanes, alkenes and alkynes
Alkaloids – a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms that are often derived from plants, e.g. quinine, morphine, curare
Alkali – a compound that forms hydroxide ions when it is dissolved in water
Amalgam – an alloy of mercury with another metal or metals
Amide – any compound derived from ammonia by substitution of an acid radical for hydrogen
Amines – organic compounds and functional groups that contain a basic nitrogen atom with a lone pair
Amorphous – existing in a non-crystalline or disordered form
Amphoteric species – a molecule or ion that can react as an acid as well as a base
Anion – a negatively charged ion in an electrolyte solution, attracted to the anode under the influence of a difference in electrical potential
Anode – electrode where oxidation occurs, i.e., the positive electrode in an electrolytic cell or a storage battery. An anode is an electrode through which electric current flows into a polarized electrical device
Anodizing – a process of coating aluminum by anodic treatment resulting in a thin film of aluminum oxide of extreme hardness
Aromatic compound – carbon atoms are arranged in a ring structure with clouds of delocalized electrons above and below, e.g. benzene
Aromaticity – a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit stabilization stronger than would be expected
Atom – the smallest part in an element that can take part in a chemical process
Avogadro’s number – 6.023 x 1023 mol-1 permits the calculation of amount of pure substance (mole)
Avogadro’s number – the number of atoms needed such that the number of grams of a substance equals the atomic mass of the substance
Base – any of a class of compounds that react with acids and some metals to form salts. A base that dissolves in water is an alkali
Boiling point – the temperature at which the vapour pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapour
Boiling-point elevation – the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water
Bond – the physical phenomenon of chemical substances being held together by attraction of atoms to each other through sharing, as well as exchanging, of electrons or electrostatic forces
Brownian motion – the random movement of solid particles suspended in a liquid or gas, caused by collisions between these particles and the molecules of the liquid or gas
Buffer – a substance added to a solution to maintain the level of its acidity when an acid or base is added to the solution
Burette – measuring instrument consisting of a graduated glass tube with a tap at the bottom; used for titration
Calcination – (also referred to as calcining) is a thermal treatment process applied to ores and other solid materials in order to bring about a thermal decomposition, phase transition, or removal of a volatile fraction, e.g. the decomposition of calcium carbonate (limestone) to calcium oxide (lime) and carbon dioxide, in order to produce cement
Carbonyl group – a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. Grignard reaction – Grignard reagents add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds
Catalysis – the change in rate of a chemical reaction due to the participation of a catalyst
Cathode – a negatively charged electrode where reduction occurs, as of an electrolytic cell, a storage battery, or an electron tube. A cathode is an electrode through which electric current flows out of a polarized electrical device
Cation – a positively charged ion that migrates through the electrolyte toward the cathode under the influence of a potential gradient. Ammonium cation is a polyatomic ion NH4+
Hydronium cation is the positively charged polyatomic ion with the formula H3O+
Cermet – a composite material composed of ceramic (cer) and metallic (met) materials
Chemical equilibrium – the state in which both reactants and products are present at concentrations which have no further tendency to change with time
Chiral – used to describe an object that is non-superimposable on its mirror image. In terms of chemistry, such objects are usually molecules
Chromatography – any of various techniques for the separation of complex mixtures that rely on the differential affinities of substances for a gas or liquid mobile medium and for a stationary adsorbing medium through which they pass
Colloid – a stable system of small particles dispersed in something else
Composite materials – often shortened to composites, are engineered or naturally occurring materials made from two or more constituent materials with significantly different physical or chemical properties, e.g. carbon fibre reinforced plastic
Compound – a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. The atoms of the elements combine to form molecules
Condenser – a device or unit used to condense a substance from its gaseous to its liquid state, typically by cooling it. Liebig condenser is the most basic water-cooled design of condenser
Conduction band – the range of electron energies enough to free an electron from binding with its atom to move freely within the atomic lattice of the material as a 'delocalized electron'. Various materials may be classified by their band gap: this is defined as the difference between the valence and conduction bands
Covalent bond – characterized by the sharing of pairs of electrons between atoms, and other covalent bonds. In short, the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding
Cracking – the process of breaking down chemical compounds using heat
Space groups and crystals are divided into seven crystal systems according to their point groups, and into seven lattice systems according to their Bravais lattices
Deliquescent materials – substances (mostly salts) that have a strong affinity for moisture and will absorb relatively large amounts of water from the atmosphere if exposed to it, forming a liquid solution
Delocalized electrons – electrons in a molecule that are not associated with a single atom or one covalent bond
Diatomic molecules – molecules composed only of two atoms, of either the same or different chemical elements. Seven elements exist in the diatomic state in the liquid and solid forms: H2, N2, O2, F2, Cl2, Br2, and I2
Diffusion – the movement of molecules from a high concentration to a low concentration
Dipole – the separation of charges within a molecule between two atoms
Dissolution – the process by which a solute forms a solution in a solvent, e.g. making a saline water solution by dissolving table salt in water. The salt is the solute and the water the solvent
Distillation – a method of separating mixtures based on differences in volatility of components in a boiling liquid mixture
Efflorescence – the loss of water (or a solvent) of crystallization from a hydrated or solvated salt to the atmosphere on exposure to air
Electrochemical cell – consists of two half-cells. Each half-cell consists of an electrode, and an electrolyte. A salt bridge is often employed to provide ionic contact between two half-cells with different electrolytes, to prevent the solutions from mixing and causing unwanted side reactions
Electrode – either of the conductors or terminals by which a current enters of leaves a conducting substance
Electrolysis – chemical change, especially decomposition, produced in an electrolyte by an electric current
Electrolyte – a solution that conducts electricity
Electrolytic cell – a cell containing an electrolyte through which an externally generated electric current is passed by a system of electrodes in order to produce an electrochemical reaction, used in e.g. electroplating
Electronegativity – a chemical property that describes the ability of an atom to attract electrons towards itself in a covalent bond. First proposed by Linus Pauling in 1932
Element – a substance that cannot be resolved into a simpler substance by a chemical process (first described by Aristotle)
Emission spectrum – the spectrum of frequencies of electromagnetic radiation emitted due to an atom's electrons making a transition from a high energy state to a lower energy state. The energy of the emitted photon is equal to the energy difference between the two states
Emulsifier – a substance that stabilizes an emulsion by increasing its kinetic stability, e.g. egg yolk, honey, mustard
Emulsion – a suspension of small globules of one liquid in a second liquid with which the first will not mix
Endothermic – chemical reaction accompanied by the absorption of heat
Ester – any of a class of organic compounds corresponding to the inorganic salts and formed from an organic acid and an alcohol
Eutectic system – a mixture of chemical compounds or elements that has a single chemical composition that solidifies at a lower temperature than any other composition
Exothermic – chemical reaction accompanied by the release of heat
Filtration – the mechanical or physical operation which is used for the separation of solids from fluids (liquids or gases) by interposing a medium through which only the fluid can pass
Flame test – an analytic procedure used to detect the presence of certain elements, primarily metal ions, based on each element's characteristic emission spectrum. The colour of flames in general also depends on temperature
Foam – a substance that is formed by trapping many gas bubbles in a liquid or solid
Flocculation – process wherein colloids come out of suspension in the form of floc or flakes by the addition of a clarifying agent
Fractional distillation – the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which one or more fractions of the compound will vapourize
Functional groups – specific groups of atoms or bonds within molecules that are responsible for the characteristic chemical reactions of those molecules
Galvanization – the process of applying a protective zinc coating to steel or iron, to prevent rusting
Group – two or more atoms within a compound that are bound together and act as a unit in chemical reactions
Hybridization – the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds
Hydrogenation – the chemical adding of hydrogen to a material. It is used to turn liquid oil into solid saturated fat
Hydrogen bond – the electromagnetic attractive interaction between polar molecules in which hydrogen is bound to a highly electronegative atom, such as nitrogen, oxygen or fluorine. The hydrogen bond is stronger than a van der Waals interaction, but weaker than covalent or ionic bonds
Hydrolysis – a chemical reaction during which molecules of water are split into hydrogen cations (H+) and hydroxide anions (OH−) in the process of a chemical mechanism
Hygroscopy – the ability of a substance to attract and hold water molecules from the surrounding environment through either absorption or adsorption
Immiscible – liquids that are not soluble in each other form two distinct layers (like water and oil)
Ion – an atom or a group of atoms that has acquired a net electric charge by gaining or losing one or more electrons
Ionic bond – involves a metal and a nonmetal ion through electrostatic attraction. The metal donates one or more electrons, forming a positively charged ion or cation with a stable electron configuration. These electrons then enter the non metal, causing it to form a negatively charged ion or anion which also has a stable electron configuration. The electrostatic attraction between the oppositely charged ions causes them to come together and form a bond
Ionic compound – a chemical compound in which ions are held together in a lattice structure by ionic bonds, e.g. sodium chloride, where equal numbers of positively-charged sodium ions and negatively-charged chlorine ions are present
Ionization – the formation of or separation into ions by heat, electrical discharge, radiation, or chemical reaction
Isomers – molecules with the same chemical formula and often with the same kinds of bonds between atoms, but in which the atoms are arranged differently. They have different structural formulae, e.g. sucrose and lactose. Isomerism was first noticed in 1827 by Friedrich Woehler
Ketone – an organic compound which features a carbonyl group (C=O) bonded to two other carbon atoms. Examples include many sugars and the industrial solvent acetone
Latex – the stable dispersion (emulsion) of polymer microparticles in an aqueous medium
Le Chatelier's principle can be used to predict the effect of a change in conditions on a chemical equilibrium. It can be summarized as ‘If a chemical system at equilibrium experiences a change in concentration, temperature, volume, or partial pressure, then the equilibrium shifts to counteract the imposed change and a new equilibrium is established’. The principle is named after Henry Louis Le Chatelier
Ligand – an ion or molecule that binds to a central metal atom
Limiting reagent – also known as the limiting reactant, is the substance which is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent since the reaction cannot proceed further without it
Lipid – any of a group of organic compounds, including the fats, oils, waxes, sterols, and triglycerides, that are insoluble in water
Liquid air – air that has been cooled to very low temperatures (cryogenic temperatures), so that it has condensed into a pale blue mobile liquid. To protect it from room temperature, it must be kept in a vacuum insulated flask
Lone pair – a valence electron pair without bonding or sharing with other atoms
Mass spectrometry – an analytical technique that measures the mass-to-charge ratio of charged particles. It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules
Metal – an element, compound, or alloy characterized by high electrical conductivity. In a metal, atoms readily lose electrons to form positive ions (cations). Those ions are surrounded by delocalized electrons, which are responsible for the conductivity. The high density of most metals is due to the tightly packed crystal lattice of the metallic structure
Metallic bonding – constitutes the electrostatic attractive forces between the delocalized electrons, called conduction electrons, gathered in an electron cloud and the positively charged metal ions
Miscible – if two liquids dissolve in each other they form a single continuous layer (like water and alcohol)
Mixture – a material system made up of two or more different substances which are mixed but are not combined chemically. A mixture refers to the physical combination of two or more substances on which the identities are retained and are mixed in the form of solutions, suspensions, and colloids
Molality – the amount of substance (in mol) of solute, divided by the mass (in kg) of the solvent
Molar concentration – the amount of a constituent (usually measured in moles) divided by the volume of the mixture. It is also called molarity
Mole – an Avogadro's number of substance. The mole is defined as the amount of substance that contains as many elementary entities (e.g., atoms, molecules, ions, electrons) as there are atoms in 0.012 kg of the isotope carbon-12 (12C)
Molecule – the smallest particle of a compound that can exist independently and retain its properties. Has at least two atoms held together by covalent bonds
Oxidation – the loss of electrons / hydrogen or gain of oxygen / increase in oxidation state by a molecule, atom or ion
Oxidation state – often called the oxidation number, is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by integers. In inorganic nomenclature the oxidation state is determined and expressed as an oxidation number represented by a Roman numeral placed after the element name e.g. copper [II] oxide
Oxidizing agent – (also oxidizer) is the element or compound in a redox reaction that accepts an electron from another species. Because the oxidizing agent is gaining electrons (and is thus often called an electron acceptor), it is said to have been reduced. The oxidizing agent itself is reduced, as it is taking electrons onto itself, but the reactant is oxidized by having its electrons taken away by the oxidizing agent
pH – potential of hydrogen atoms. A measure of the acidity or basicity of a solution. It approximates but is not equal to p[H], the negative logarithm (base 10) of the molar concentration of dissolved hydronium ions (H3O+). The concept of p[H] was first introduced by Danish chemist Soren Sorensen in 1909. Acid rain – pH < 5.6, pH of blood is 7.4, pH of urine is 6, pH of gastric acid is 1
Phlogiston theory – postulated a fire-like element called phlogiston, contained within combustible bodies, is released during combustion. It was first stated in 1667 by Johann Joachim Becher. Phlogisticated substances are substances that contain phlogiston and dephlogisticate when burned
Photochemical reaction – any chemical reaction caused by absorption of light (including visible, ultraviolet, and infrared)
Photochromism – a reversible transformation of a chemical species induced in one or both directions by absorption of electromagnetic radiation between two forms, A and B, having different absorption spectra
Pipette – glass or transparent plastic tube used in measuring or transferring small amounts of liquid
Plastic – a type of polymer with low elasticity
Polar bond – a type of covalent bond between two atoms in which electrons are shared unequally. Because of this, one end of the molecule has a slightly negative charge and the other a slightly positive charge, e.g. H2O
Polarity – refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Due to the polar nature of the water molecule itself, polar molecules are generally able to dissolve in water
Polarization – the partial or complete polar separation of positive and negative electric charge
Polyatomic ion – also known as a molecular ion, is an ion composed of two or more atoms covalently bonded or of a metal complex that can be considered to be acting as a single unit, e.g. ammonium ion NH4+
Polymerization – a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains
Precipitate – a solid that is formed as a result of a precipitation reaction, e.g. mixing silver nitrate and sodium chloride in water will cause silver chloride to precipitate out of solution as a solid
Radicals – (often referred to as free radicals) are atomic or molecular species with unpaired electrons on an otherwise open shell configuration. These unpaired electrons are usually highly reactive, so radicals are likely to take part in chemical reactions
Radiometric dating – a technique used to date materials based on a knowledge of the decay rates of naturally occurring isotopes, and the current abundances. It is our principal source of information about the age of the Earth and a significant source of information about rates of evolutionary change
Radionuclide – an atom with an unstable nucleus, characterized by excess energy. Often referred to as radioactive isotope or radioisotope
Reactant – a substance that is consumed in the course of a chemical reaction
Reactions – usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants
Reagent – a substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs
Redox – (portmanteau of reduction and oxidation) reactions include all chemical reactions in which atoms have their oxidation state changed
Reduction – the gain of electrons / hydrogen or a loss of oxygen / decrease in oxidation state by a molecule, atom or ion
Resonance – a way of describing delocalized electrons within certain molecules where the bonding ‘hovers’, e.g. between single and double
Reversible reaction – results in an equilibrium mixture of reactants and products
Saturated compound – a compound in which all the carbon atoms are joined by single bonds, making it incapable of combining with additional atoms
Saturation – the point at which a solution of a substance can dissolve no more of that substance and additional amounts of it will appear as a separate phase (as a precipitate if solid)
Side chain – a chemical group that is attached to a core part of the molecule called main chain or backbone. The placeholder R is often used as a generic placeholder side chains in chemical structure diagrams
Solution – a homogeneous mixture of two or more substances; frequently (but not necessarily) a liquid solution. The solute is dissolved in the solvent
Spectator ions – remain unchanged on both sides of an equation. They simply ‘watch’ the other ions react, hence the name
Stereoisomers – isomeric molecules that have the same molecular formula and sequence of bonded atoms (constitution), but that differ only in the 3D orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections and/or their order differ(s) between different atoms/groups. In stereoisomers, the order and bond connections of the constituent atoms remain the same, but their orientation in space differs
Stoichiometry – deals with the relative quantities of reactants and products in chemical reactions. Stoichiometry can be used to determine quantities such as the amount of products that can be produced with given reactants. Reaction and composition – two types of stoichiometry
Stereochemistry – involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. Also known as 3D chemistry
Suspension – a mixture in which fine particles are suspended in a fluid where they are supported by buoyancy
Thermoplastic – a polymer that turns to a liquid when heated and freezes to a very glassy state when cooled sufficiently. Thermoplastic polymers differ from thermosetting polymers (Bakelite) in that they can be remelted and remoulded
Tincture – a solution that has alcohol as its solvent
Titration – used to find the concentration of a substance (titrand) in a solution by adding measured quantities of another substance (titrant) that is known to react with it, until the reaction reaches a known endpoint
Universal indicator – a pH indicator composed of a solution of several compounds that exhibits several smooth colour changes over a pH value range from 1-14 to indicate the acidity or alkalinity of solutions
Unsaturated compound – a compound in which two or more of the carbon atoms are joined by double or triple bonds, making it capable of combining with additional atoms
Valence electrons – the outermost electrons of an atom, which are important in determining how the atom reacts chemically with other atoms
Valency – the combining capacity of an atom or radical determined by the number of electrons that it will lose, add, or share when it reacts with other atoms. A univalent (monovalent) atom, ion or group has a valence of one and thus can form one covalent bond. A divalent ion or molecule has a valence of two and thus can form two bonds with other ions or molecules
Van der Waals’ force – the sum of the attractive or repulsive forces between molecules (or between parts of the same molecule) other than those due to covalent bonds, the hydrogen bonds, or the electrostatic interaction of ions with one another or with neutral molecules or charged molecules. The term includes: force between two permanent dipoles (Keesom force); force between a permanent dipole and a corresponding induced dipole (Debeye force); force between two instantaneously induced dipoles (London dispersion force
Vapour pressure – the pressure exerted by a vapour in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system
Voltaic cell – an electrochemical cell that generates an electromotive force by an irreversible conversion of chemical to electrical energy, e.g. a battery
Volatility – the tendency of a substance to vaporize. Volatility is directly related to a substance's vapour pressure. At a given temperature, a substance with higher vapour pressure vaporizes more readily than a substance with a lower vapour pressure
Vulcanisation – a chemical process for converting rubber or related polymers into more durable materials via the addition of sulfur or other equivalent ‘curatives’
Zwitterion – a compound with a positive and a negative charge on different atoms and a total net charge of zero