Physical World/Physics
Atomic physics
Plum pudding model of the atom by J. J. Thomson, who discovered the electron in 1897, was proposed in 1904 before the discovery of the atomic nucleus. In this model, the atom is composed of electrons (which Thomson still called ‘corpuscles’) surrounded by a soup of positive charge to balance the electrons' negative charges, like negatively-charged ‘plums’ surrounded by positively-charged ‘pudding’
Geiger–Marsden experiment (also called the Rutherford gold foil experiment) was an experiment to prove the structure of the atom. The unexpected results of the experiment demonstrated for the first time the existence of the atomic nucleus, leading to the downfall of the plum pudding model of the atom
Electron shell – an orbit followed by electrons around an atom nucleus. Because each shell can contain only a fixed number of electrons, each shell is associated with a particular range of electron energy, and thus each shell must fill completely before electrons can be added to an outer shell. The electrons in the outermost shell determine the chemical properties of the atom
The electron shells are labeled K, L, M, N, O, P, and Q; or 1, 2, 3, 4, 5, 6, and 7; going from innermost shell outwards
Electron configuration was first conceived of under the Bohr model of the atom. An electron shell is the set of allowed states electrons may occupy which share the same principal quantum number, n (the number before the letter in the orbital label). An atom's nth electron shell can accommodate 2n2 electrons, e.g. the first shell can accommodate 2 electrons, the second shell 8 electrons, and the third shell 18 electrons
Each shell is composed of one or more subshells, which are themselves composed of atomic orbitals
A subshell is the set of states defined by a common azimuthal quantum number, l, within a shell. The values l = 0, 1, 2, 3 correspond to the s, p, d, and f labels, respectively. The maximum number of electrons which can be placed in a subshell is given by 2(2l + 1). This gives two electrons in an s subshell, six electrons in a p subshell, ten electrons in a d subshell and fourteen electrons in an f subshell
The outermost electron shell is often referred to as the ‘valence shell’ and determines the chemical properties
Atomic orbital – a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which correspond to the electron's energy, angular momentum, and an angular momentum vector component, respectively. Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations. They are derived from the characteristics of their spectroscopic lines: sharp, principal, diffuse, and fundamental
Electrons can jump from one orbit to another, gaining or losing electromagnetic radiation with a frequency (ν) proportional to the energy difference (E) according to the Planck relation
Aufbau principle – electrons fill orbitals starting at the lowest available (possible) energy levels before filling higher levels (e.g. 1s before 2s). The number of electrons that can occupy each orbital is limited by the Pauli exclusion principle. The order in which these orbitals are filled is given by the Madelung rule
Nuclear physics
Rutherford fired alpha particles (helium nuclei) through nitrogen gas, which was turned into oxygen, in 1917. Rutherford named the hydrogen nucleus released a ‘photon’
Rutherford is widely credited with first splitting the atom in 1917, and leading the first experiment to ‘split the nucleus’ in a controlled manner by two students under his direction, John Cockcroft and Ernest Walton in 1932. They bombarded lithium with high energy neutrons, electrons and protons and succeeded in transmuting it into helium and other chemical elements
In 1900, Ernest Rutherford and Paul Villard separated radiation into three types: alpha, beta, and gamma, based on penetration of objects and ability to cause ionization. Alpha rays were defined by Rutherford as those having the lowest penetration of ordinary objects
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is produced in the process of alpha decay. A positively charged particle that is the nucleus of the helium atom; emitted from natural or radioactive isotopes. Can be blocked by a sheet of paper
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms (or 'decays') into an atom with a mass number 4 less and atomic number 2 less. For example, uranium-238 decays through α-particle emission to form thorium-234
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. Can be blocked by a sheet of aluminium
There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus (β−), while in the case of a positron emission as beta plus (β+). In electron emission, an electron antineutrino is also emitted, while positron emission is accompanied by an electron neutrino. Beta decay is mediated by the weak force
Beta minus decay – neutron changes into a proton by emitting an electron and an antineutrino via an intermediate W- boson. The original element decays into a new element with an unchanged mass number but an atomic number that has increased by one. For example, carbon-14 decays into nitrogen-14
Beta plus decay – proton changes into a neutron by emitting a positron and a neutrino. The resulting element has an atomic number that has decreased by one. The weak interaction converts a proton into a neutron by converting an up quark into a down quark by having it emit a W+ or absorb a W-. This happens in the sun as the first stage of fusion
Tritium decays into helium-3 by beta decay
Theory of beta decay published by Fermi in 1934
Gamma rays – emitted from the nucleus during radioactive decay
Gamma radiation, also known as gamma rays, is electromagnetic radiation of high frequency (very short wavelength). They are produced by sub-atomic particle interactions such as electron-positron annihilation. Because they are a form of ionizing radiation, gamma rays can cause serious damage when absorbed by living tissue. Can be blocked by a block of concrete
Paul Villard discovered gamma rays in 1900 while studying the radiation from radium
Gamma rays were named by Ernest Rutherford
In the past, the distinction between X-rays and gamma rays was based on energy (or equivalently frequency or wavelength), with gamma rays being considered a higher-energy version of X-rays. However, modern high-energy X-rays produced by linear accelerators (‘linacs’) usually have higher energy than gamma rays produced by radioactive gamma decay. Because of this broad overlap in energy ranges, the two types of electromagnetic radiation are now usually defined by their origin: X-rays are emitted by electrons (either in orbitals outside of the nucleus, or while being accelerated to produce Bremsstrahlung-type radiation), while gamma rays are emitted by the nucleus or from other particle decays or annihilation events
Chain reaction – a multistage nuclear reaction, especially a self-sustaining series of fissions in which the release of neutrons from the splitting of one atom leads to the splitting of others
Enrico Fermi obtained the first chain reaction in 1942 at the University of Chicago, beneath the football stadium
Atomic bomb – fire a neutron at a uranium atom. This releases the neutrons, leading to a chain reaction. Realised by Leo Szilard. Application of E=mc2
Nuclear fission – a nuclear reaction in which the nucleus of an atom splits into smaller parts (lighter nuclei), often producing free neutrons and photons (in the form of gamma rays), as well. Fission of heavy elements is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments. The atomic nucleus is usually uranium-235 or plutonium-239
Molten salt reactor (MSR) is a class of nuclear fission reactors in which the primary coolant, or even the fuel itself, is a molten salt mixture
A breeder reactor is a nuclear reactor capable of generating more fissile material than it consumes. Two types – fast breeder and thermal breeder
Nuclear fusion – the process by which multiple atomic nuclei join together to form a single heavier nucleus. It is accompanied by the release or absorption of large quantities of energy. Fusion power advocates commonly propose the use of deuterium, or tritium, both isotopes of hydrogen, as fuel
The fusion of two nuclei with lower masses than iron (which, along with nickel, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission. This means that fusion generally occurs for lighter elements only, and likewise, that fission normally occurs only for heavier elements
Fusion reactors fuse deuterium with tritium creating helium-4, freeing a neutron, and releasing 17.6MeV of energy
Mark Oliphant fused two deuterium nuclei to make helium in the first demonstration of nuclear fusion
The Sun’s energy mostly comes from the nuclear fusion of deuterium or tritium to make helium
At the heart of the Sun:
1. Two protons combine to make a deuteron, releasing a positron and a neutrino
2. Deuteron links with a proton, forming 3He
3. Two 3He combine to make one 4He, releasing two spare protons
Cold fusion – a hypothetical form of nuclear fusion occurring without the use of extreme temperature or pressure
Radiation – electromagnetic radiation emitted from a material which is due to the heat of the material. The process in which energy in the form of rays of light, heat, etc. is sent out through space from atoms and molecules as they undergo internal change
Ionizing radiation consists of subatomic particles or electromagnetic waves that are energetic enough to detach electrons from atoms or molecules, thus ionizing them
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting ionizing particles or radiation
Radioactive activity is measured in bequerels
Radioactive exposure is measured in rontgens
Absorbed dose of radiation is measured in grays
Dose equivalent is measured in sieverts
Radioactivity – the spontaneous emission of a stream of particles or electromagnetic rays in nuclear decay
Radiance and spectral radiance are radiometric measures that describe the amount of radiation such as light or radiant heat that passes through or is emitted from a particular area, and falls within a given solid angle in a specified direction
Cherenkov radiation is electromagnetic radiation emitted when a charged particle passes through an insulator at a speed greater than the speed of light in that medium. The characteristic ‘blue glow’ of nuclear reactors is due to Cherenkov radiation
Uranium 235 and 238 are the principal isotopes. Uranium 238 decays to Plutonium 239
Plutonium-239 has a half-life of 24,100 years
Enriched uranium – a kind of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Natural uranium is 98% U-238. The 238U remaining after enrichment is known as depleted uranium
Turning raw uranium has two steps – conversion (solid uranium, known as yellow cake, is heated to convert it into a gap), and enrichment (uranium gas is spun in centrifuges to make the gas rich enough in U-235 to achieve the ‘critical mass’ that can sustain a nuclear reaction)
Uranium is the main source of nuclear energy
Uranium decays to lead-206
Island of stability – a set of as-yet undiscovered heavier isotopes of transuranium elements which are theorized to be much more stable than some of those closer in atomic number to uranium
Nuclear weapons
Weapons whose explosive output is exclusively from fission reactions are commonly referred to as atomic bombs or A-bombs. In fission weapons, a mass of fissile material (enriched uranium or plutonium) is assembled into a supercritical mass – the amount of material needed to start an exponentially growing nuclear chain reaction
Thermonuclear weapon uses the heat generated by a fission bomb to compress a nuclear fusion stage. This indirectly results in a greatly increased energy yield. It is colloquially referred to as a hydrogen bomb or H-bomb because it employs hydrogen fusion, though in most applications the majority of its destructive energy comes from uranium fission, not hydrogen fusion alone
Neutron bomb or enhanced radiation weapon (ERW) is a type of thermonuclear weapon designed specifically to release a large portion of its energy as energetic neutron radiation (fast neutrons) rather than explosive energy. Although their extreme blast and heat effects are not eliminated, it is the enormous radiation released by ERWs that is meant to be a major source of casualties
Bubble chamber – a vessel filled with a superheated transparent liquid (most often liquid hydrogen) used to detect electrically charged particles moving through it. Invented by Donald A. Glaser in 1952
Cloud chamber, also known as the Wilson chamber, is used for detecting particles of ionizing radiation. Vapour inside the chamber is cooled, forming a cloud of droplets on the charged particles of the substance being observed. Invented by Charles Wilson in 1911
Diffusion cloud chamber developed by Alexander Langsdorf. Uses dry ice
Cloud chambers work on the same principles as bubble chambers, only they are based on supersaturated vapour rather than superheated liquid
Electron capture – a process in which a proton-rich nuclide absorbs an inner atomic electron, thereby changing a nuclear proton to a neutron and simultaneously causing the emission of an electron neutrino. It is sometimes called inverse beta decay
Half-life is the period of time it takes for a substance undergoing decay to decrease by half. Half-life is used to describe a quantity undergoing exponential decay, and is constant over the lifetime of the decaying quantity
Liquid drop model – a model in nuclear physics which treats the nucleus as a drop of incompressible nuclear fluid, first proposed by George Gamow and developed by Niels Bohr and John Archibald Wheeler. The fluid is made of nucleons (protons and neutrons), which are held together by the strong nuclear force
Moseley’s Law concerns the characteristic X-rays that are emitted by atoms. It is important in quantitatively justifying the conception of the nuclear model of the atom, with all, or nearly all, positive charges of the atom located in the nucleus, and associated on an integer basis with atomic number
Nuclear potential energy – the potential energy of the particles inside an atomic nucleus. The nuclear particles are bound together by the strong nuclear force
Nuclide – an atomic species characterized by the specific constitution of its nucleus, i.e., by its number of protons, its number of neutrons, and its nuclear energy state. Primordial nuclides, also known as primordial isotopes, are nuclides found on the Earth that have existed in their current form since before Earth was formed
Semi-empirical mass formula (SEMF) is used to approximate the mass and various other properties of an atomic nucleus. As the name suggests, it is based partly on theory and partly on empirical measurements. The theory is based on the liquid drop model proposed by George Gamow
Spallation – the process in which a heavy nucleus emits a large number of nucleons as a result of being hit by a high-energy particle, thus greatly reducing its atomic weight
Wigner effect – the displacement of atoms in a solid caused by neutron radiation. Indirect cause of the Windscale fire in 1957
Geiger–Muller counter, also called a Geiger counter – measures the level of ionizing radiation. An inert gas-filled tube briefly conducts electricity when a particle or photon of radiation makes the gas conductive. The tube amplifies this conduction and outputs a current pulse, which is then often displayed by a needle or lamp and/or audible
Tokamak – a machine producing a toroidal (doughnut-shaped) magnetic field for confining a plasma which is characterized by azimuthal (rotational) symmetry and the use of a plasma-borne electric current to generate the helical component of the magnetic field necessary for stable equilibrium. It is one of several types of magnetic confinement devices and the leading candidate for producing fusion energy
ITER (originally the International Thermonuclear Experimental Reactor) is an international tokamak (magnetic confinement fusion) research/engineering project that could help to make the transition from today's studies of plasma physics to future electricity-producing fusion power plants. Construction of the ITER complex on the site in Cadarache in Provence-Alpes-Côte-d'Azur began in 2008. ITER is intended to be an experimental step between today's studies of plasma physics and future electricity-producing fusion power plants. Fuelled by deuterium and tritium
JET, the Joint European Torus, is the worlds largest magnetic confinement plasma physics experiment located in Culham, Oxfordshire. Based on a tokamak design
National Ignition Facility, or NIF, is a laser-based inertial confinement fusion (ICF) research device located at the Lawrence Livermore National Laboratory
ZETA – Zero Energy Thermonuclear Assembly, devised in 1957 at Harwell, was a major experiment in the early history of fusion power research
Particle physics
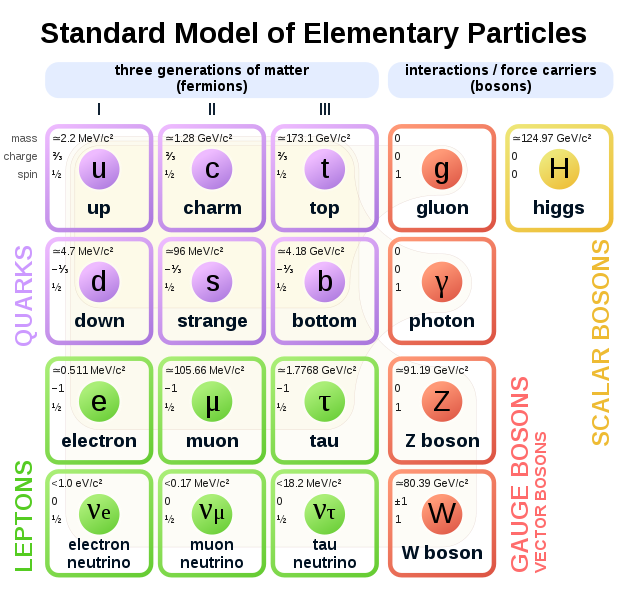
The Standard Model of particle physics is a theory concerning the electromagnetic, weak, and strong nuclear interactions, as well as classifying all the subatomic particles known. The Standard Model recognizes 24 different fermions – six quarks, six leptons, and their antiparticles
Fermion – any particle characterized by Fermi–Dirac statistics with half integer spin. These particles obey the Pauli exclusion principle. Fermions include all quarks and leptons, as well as any composite particle made of an odd number of these, such as all baryons and many atoms and nuclei. Fermions differ from bosons, which obey Bose–Einstein statistics
Quarks are spin ½ particles, implying that they are fermions
Murray Gell-Mann introduced a classification scheme for hadrons, known as the Eightfold Way, because of the octets of particles in the classification. In 1964, Gell-Mann and, independently, George Zweig went on to postulate the existence of quarks
Top quark found by the Tevatron. Last quark to be found, in 1995. Top quark has by far the greatest mass (173 GeV). Lifetime of top quark is too short to form hadrons
Flavours of quark – up, down, top, bottom, strange and charm
Up, charm, and top quarks (collectively referred to as up-type quarks) have a charge of +2⁄3, while down, strange, and bottom quarks (down-type quarks) have −1⁄3. Antiquarks have the opposite charge to their corresponding quarks
Truth and beauty – old names for top and bottom quarks
The defining property of the quarks is that they carry colour charge, and hence, interact via the strong interaction
The quark model is a classification scheme for hadrons in terms of their valence quarks – the quarks and antiquarks which give rise to the quantum numbers of the hadrons
Sheldon Glashow and James Bjorken predicted the existence of the charm quark
Leptons – fermions with a spin ½ particles. Each lepton has a corresponding antilepton. Leptons are not subject to the strong nuclear force and do not carry a colour charge. The three neutrinos do not carry electric charge either, so their motion is directly influenced only by the weak nuclear force, which makes them notoriously difficult to detect. However, by virtue of carrying an electric charge, the electron, muon, and tau all interact electromagnetically
Electron – a fundamental subatomic particle that carries a negative electric charge. It is a spin-½ lepton that participates in electromagnetic interactions
J.J. Thomson is credited for the discovery of the electron, in 1897
Electron – only fundamental particle discovered before 1930
Muon – elementary particle in the lepton family (not a meson), having a mass 209 times that of the electron, and a negative electric charge
Tau – elementary particle in the lepton family with a negative electric charge and a mass 3477 times that of the electron. The only lepton that can decay into hadrons
Neutrino – electrically neutral lepton. An elementary particle with zero charge and a tiny mass which has never been measured. Three types – electron neutrino, muon neutrino, and tau neutrino
In the 1956 Cowan–Reines neutrino experiment, antineutrinos created in a nuclear reactor by beta decay reacted with protons producing neutrons and positrons. This was the first experiment that detected the neutrino
Neutrinos change – or oscillate – between the three flavours
Neutrino – postulated 20 years before discovery. Interacts weakly with matter
Neutrino was first postulated in 1930 by Wolfgang Pauli to preserve the conservation of energy, conservation of momentum, and conservation of angular momentum in beta decay
Boson – a particle that follows Bose-Einstein statistics. Examples of bosons include fundamental particles such as photons, gluons, and W and Z bosons, the Higgs boson, and the still-theoretical graviton of quantum gravity; composite particles (e.g. mesons and stable nuclei of even mass number such as deuterium; and some quasiparticles
Bosons have integer spin
Gauge bosons are bosonic particles that act as carriers of the fundamental forces of nature. In the Standard Model, there are three kinds of gauge bosons: photons, W and Z bosons, and gluons. Each corresponds to one of the three Standard Model interactions: photons are gauge bosons of the electromagnetic interaction, W and Z bosons carry the weak interaction, and the gluons carry the strong interaction
W and Z bosons were discovered at CERN in 1983 in the Super Proton Synchrotron
W boson differs from the photon in two important ways – it has electric charge and a large mass. Mass of W boson is 80 times greater than that of a proton or neutron
The W bosons (W+ and W-) have a positive and negative electric charge of 1 elementary charge respectively and are each other's antiparticles
Scalar boson – a boson whose spin equals zero. The only scalar boson is the Higgs boson
Vector boson – a boson with the spin equal to 1
Gluons ‘hold quarks together’. They act as the exchange particles (or gauge bosons) for the colour force between quarks, analogous to the exchange of photons in the electromagnetic force between two charged particles
There are eight independent types of gluon. Quarks carry three types of colour charge; antiquarks carry three types of anticolour. Gluons act as the exchange particles (or gauge bosons) for the strong force between quarks
Photon – the quantum of electromagnetic energy, regarded as a discrete particle having zero mass, no electric charge, and an indefinitely long lifetime. Photons can travel through a vacuum
When photons are scattered from an atom or molecule, most photons are elastically scattered (Rayleigh scattering), such that the scattered photons have the same energy (frequency and wavelength) as the incident photons
Higgs boson was postulated by Peter Higgs in 1964 as a way to give inertia to particles. It tugs on force carriers such as the W and Z bosons and breaks the symmetry between the weak and electromagnetic forces. Known as the ‘God particle’. It would have a spin of zero and explain how particles get their mass
Higgs boson may exist near a mass of 125 GeV
Higgs boson discovery was announced at CERN on 4 July 2012. It appears to confirm the existence of the Higgs field. It would explain why some fundamental particles have mass when the symmetries controlling their interactions should require them to be massless, and why the weak force has a much shorter range than the electromagnetic force
Standard model has 61 elementary particles
Particle physics has an accepted definition for a ‘discovery’: a five-sigma level of certainty. Used to confirm discovery of Higgs boson
Colour charge is a property of quarks and gluons which are related to their strong interactions in the context of Quantum chromodynamics. The colour of quarks (red, green and blue) and gluons has nothing to do with the visual perception of colour
There are four fundamental forces within all atoms, which dictate interactions between individual particles, and the large-scale behaviour of all matter throughout the Universe. They are the strong and weak nuclear forces, the electromagnetic force and gravitation
The strong interaction (strong nuclear force) occurs between the types of subatomic particles that are made up of quarks; these include baryons and mesons. This interaction binds protons and neutrons together in the nuclei of atoms, and is carried by gluons
The weak interaction (weak nuclear force) affects quarks and leptons. It can transform neutrons into protons and vice versa, and is transmitted by intermediate vector bosons
The weak nuclear force causes the radioactive decay of certain particular atomic nuclei
Electromagnetism is the force that acts between electrically charged particles
Gravitation is the weakest force. It is carried by particles called gravitons
Graviton – hypothetical elementary particle that mediates the force of gravity in the framework of quantum field theory. If it exists, the graviton must be massless and must have a spin of 2. It would be a gauge boson
Electroweak interaction – the unified description of two of the four known fundamental interactions of nature: electromagnetism and the weak interaction. Although these two forces appear very different at everyday low energies, the theory models them as two different aspects of the same force
Grand Unified Theory (GUT) refers to any of several similar models in particle physics in which at high energy scales, the three gauge interactions of the Standard Model which define the electromagnetic, weak, and strong interactions, are merged into one single interaction characterized by a larger gauge symmetry
Theory of Everything (TOE) is a hypothetical framework that would simultaneously describe all four forces of nature that affect matter: the electromagnetic interaction, the strong and weak interactions affecting particles and nuclei, and the gravitational interaction
Hadron – a composite particle made of quarks held together by the strong force. Hadrons are categorized into two families: baryons and mesons. The best-known hadrons are protons and neutrons (both baryons), which can be found in the atomic nuclei. All hadrons except protons are unstable and undergo particle decay
Baryon – a composite subatomic particle made up of three quarks. As quark-based particles, baryons participate in the strong interaction. Baryons are strongly interacting fermions
Proton – a subatomic particle with an electric charge of one positive fundamental unit (1.602 × 10−19 coulomb) and a mass about 1836 times the mass of an electron. Protons are spin-½ fermions. Protons consist of two up quarks and one down quark
Neutron –a subatomic particle with no net electric charge and a mass slightly greater than a proton. Its spin is ½. Neutrons consist of two down quarks and one up quark
Neutron is slightly more massive than proton because down quark is slightly more massive than up quark
James Chadwick discovered the neutron in 1932 by exposing beryllium to alpha particles
Mesons are hadronic subatomic particles composed of one quark and one antiquark, bound together by the strong interaction. Mesons are bosons
From theoretical considerations, Hideki Yukawa in 1934 predicted the existence and the approximate mass of the ‘meson’ as the carrier of the nuclear force that holds atomic nuclei together
Pions are the lightest mesons and they play an important role in explaining the low-energy properties of the strong nuclear force. Short for pi meson. Zero spin. Pions are involved in holding the nucleus together
Eta and eta prime meson are mesons made of a mixture of up, down and strange quarks and their antiquarks
Kaon, or K meson, was discovered in cosmic rays in 1947
B mesons are mesons composed of a bottom antiquark and a different flavour of quark
Quarkonium – a flavorless meson whose constituents are a quark and its own antiquark
Antiparticle – a particle that has the same mass as another particle but has opposite values for its other properties. A particle and its antiparticle destroy each other on contact in the process of annihilation
Positron or antielectron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric charge of +1e, a spin of ½, and has the same mass as an electron. When a low-energy positron collides with a low-energy electron, annihilation occurs, resulting in the production of two or more gamma ray photons
Dirac predicted the existence of the positron which he interpreted in terms of what came to be called the Dirac sea (a theoretical model of the vacuum as an infinite sea of particles with negative energy)
Carl Anderson discovered the positron in 1932
Antiproton is the antiparticle of the proton. Antiprotons are stable, but they are typically short-lived since any collision with a proton will cause both particles to be annihilated in a burst of energy. The existence of the antiproton with −1 electric charge was predicted by Paul Dirac in his 1933 Nobel Prize lecture
Antineutron is the antiparticle of the neutron. It consists of one up antiquark and two down antiquarks
Axion – a hypothetical elementary particle. Possible component of cold dark matter
Compton scattering – an inelastic scattering of a photon by a free charged particle, usually an electron. It results in a decrease in energy (increase in wavelength) of the photon (which may be an X-ray or gamma ray photon), called the Compton effect. Part of the energy of the photon is transferred to the recoiling electron
CP violation – a violation of the postulated CP-symmetry (or Charge conjugation Parity symmetry): the combination of C-symmetry. CP-symmetry states that the laws of physics should be the same if a particle is interchanged with its antiparticle (C symmetry), and then its spatial coordinates are inverted (‘mirror’ or P symmetry). CP violation was discovered in 1964 in the decays of kaons
Deep inelastic scattering – fire a fast beam of electrons at protons and some are deflected by the three quarks making up the proton. Repeat the process for neutrons, which produces a different pattern for the scattered electrons, and mesons which have two scattering points
Feynman diagrams – a pictorial representation scheme for the mathematical expressions governing the behavior of subatomic particles. Feynman diagrams contain both a space axis and a time axis, and antiparticles are interpreted as moving forward in space but backward in time
Isospin – a dimensionless number related to the strong interaction
Nucleon – general term for a neutron or proton
Neutralino – hypothetical particle. As a heavy, stable particle, the lightest neutralino is an excellent candidate to comprise the universe's cold dark matter
Phonon – a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, such as solids and some liquids. Often referred to as a quasiparticle, it represents an excited state in the quantum mechanical quantization of the modes of vibrations of elastic structures of interacting particles
Quasiparticles – occur in a system such as a solid behaves as if it contained different weakly interacting particles in free space
Raman scattering or the Raman Effect is the inelastic scattering of a photon
Spin – the total angular momentum of an atomic nucleus. Particles with spin can possess a magnetic dipole moment
Spin 1/2 is actually h/2Π where h is the Planck constant
Strangeness – a property of particles, expressed as a quantum number, for describing decay of particles in strong and electromagnetic reactions, which occur in a short period of time
The terms strange and strangeness predate the discovery of the quark, and were adopted after its discovery in order to preserve the continuity of the phrase; strangeness of anti-particles being referred to as +1, and particles as −1 as per the original definition
Strangeness is conserved during the strong and the electromagnetic interactions, but not during the weak interactions. Consequently, the lightest particles containing a strange quark cannot decay by the strong interaction, and must instead decay via the much slower weak interaction
Supersymmetry – (SUSY) a symmetry that relates elementary particles of one spin to other particles that differ by half a unit of spin and are known as superpartners. In a theory with unbroken supersymmetry, for every type of boson there exists a corresponding type of fermion with the same mass and internal quantum numbers, and vice-versa. So far, there is only indirect evidence for the existence of supersymmetry
Superpartner (also sparticle) is a hypothetical elementary particle. Supersymmetry is one of the synergistic theories in current high-energy physics which predicts the existence of these ‘shadow’ particles
Fermion superpartners have ‘s’ at the front of the name, e.g. squark, slepton, selectron, sneutrino
Boson superpartners have ‘ino’ at the end of the name, e.g. photino, gluino, wino, zino
The electromagnetic radiation emitted when charged particles are accelerated in a circular path is called synchrotron radiation. It is produced in synchrotrons using bending magnets
Symmetry breaking describes a phenomenon where (infinitesimally) small fluctuations acting on a system which is crossing a critical point decide the system's fate, by determining which branch of a bifurcation is taken
Tachyon – theoretical particle faster than the speed of light. George Sudarshan was the first to propose the existence of tachyons, in 1962
Particle detectors
Cyclotron – a type of particle accelerator in which charged particles accelerate outwards from the center along a spiral path. The particles are held to a spiral trajectory by a static magnetic field and accelerated by a rapidly varying (radio frequency) electric field
Spark chambers consist of a stack of metal plates placed in a sealed box filled with an inert gas such as helium or neon. When a charged particle from a cosmic ray travels through the box, it ionizes the gas between the plates. Spark chambers were most widely used for detecting electrically charged particles from the 1930s to the 1960s and have since been superseded by other technologies such as drift chambers and silicon detectors
Synchrotron – a particular type of cyclic particle accelerator in which the magnetic field (to turn the particles so they circulate) and the electric field (to accelerate the particles) are carefully synchronized with the travelling particle beam
Bevatron (Billions of eV Synchrotron) at Lawrence Berkeley National Laboratory began operating in 1954. The antiproton was discovered there in 1955
Cosmotron was a particle accelerator, specifically a proton synchrotron, at Brookhaven National Laboratory. Its construction was approved by the U.S. Atomic Energy Commission in 1948
Cowan and Reines neutrino experiment, also known as Project Poltergeist, was performed by Clyde L. Cowan and Frederick Reines in 1956. This experiment confirmed the existence of the antineutrino
Several experiments in the 1960s, including the Homestake experiment (sometimes referred to as the Davis experiment) found that the number of electron neutrinos arriving from the Sun was between one third and one half the number predicted by the Standard Solar Model. This discrepancy, which became known as the solar neutrino problem, was resolved by discovery of neutrino oscillation and mass
LEP – Large Electron-Positron Collider, at CERN. Shutdown in 2000
LHC – 27km circular tunnel. Cost £3.5 billion
The LHC collides protons together, while the Tevatron used protons and their antimatter counterpart, antiprotons. Both experiments hunt for the Higgs by looking at what those high-energy particles decay into. At the Tevatron, the data are from the production of bottom quarks and their counterparts bottom antiquarks, whereas at the LHC the primary search is for the production of the light particles known as photons
ATLAS and CMS – general purpose particle detector experiments in LHC
LHCb (‘Large Hadron Collider beauty’) is one of seven particle physics detector experiments collecting data at the Large Hadron Collider accelerator at CERN. LHCb is a specialized b-physics experiment. Such studies can help to explain the Matter-Antimatter asymmetry of the Universe
LHC lies in a tunnel 27 km in circumference
ALICE – detector experiment at LHC studying heavy ion collisions
Oscillation Project with Emulsion-tRacking Apparatus (OPERA) is a scientific instrument for detecting tau neutrinos from muon neutrino oscillations. The experiment is a collaboration between CERN and the Laboratori Nazionali del Gran Sasso (LNGS) in Italy. In 2010, OPERA researchers observed the first tau neutrino candidate event in a muon neutrino beam. In 2011, OPERA researchers observed muon neutrinos traveling apparently at faster than lightspeed – the neutrinos arrived at CERN 60 nanoseconds early. The results were subsequently investigated and confirmed to be wrong. They were caused by a flawed optic fibre cable in OPERA receiver of the laboratory
Stanford Linear Accelerator Centre has the longest linear accelerator in the world (two miles) and has been operational since 1966
Sudbury Neutrino Observatory is located about 2 km underground in a mine in Ontario. The detector was designed to detect solar neutrinos through their interactions with a large tank of heavy water
Superconducting Super Collider (SSC) (also nicknamed the Desertron) was a particle accelerator complex under construction in the vicinity of Waxahachie, Texas, that was set to be the world's largest and most energetic. The project was cancelled in 1993 due to budget problems
Tevatron (Tera electron volt synchrotron) is a circular particle accelerator at the Fermi National Accelerator Laboratory (also known as Fermilab), just east of Batavia, Illinois. The Tevatron ceased operations in September 2011, due to budget cuts
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory deals with physical phenomena at nanoscopic scales, where the action is on the order of the Planck constant. The name derives from the observation that some physical quantities can change only in discrete amounts (Latin quanta), and not in a continuous way. Quantum mechanics provides a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter
Black hole information paradox – results from the combination of quantum mechanics and general relativity. It suggests that physical information could disappear in a black hole, allowing many physical states to evolve into the same state
Bohr model, or Rutherford-Bohr model, devised by Niels Bohr, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus – similar in structure to the solar system, but with electrostatic forces providing attraction, rather than gravity
In 1912 Niels Bohr joined Ernest Rutherford at Manchester University and he adapted Rutherford's nuclear structure to Max Planck's quantum theory and so obtained a theory of atomic structure which, with later improvements, mainly as a result of Heisenberg's concepts, remains valid to this day
Casimir effect – explains why two uncharged metallic plates in a vacuum, placed a few micrometers apart have a force between them
Complementarity – objects governed by quantum mechanics, when measured, give results that depend inherently upon the type of measuring device used, and must necessarily be described in classical mechanical terms
Copenhagen interpretation – in 1927 Niels Bohr combined Heisenberg’s uncertainty principle with Schrodinger’s wave equation to explain how an observer’s intervention means that there are things we can never know. It requires wavefunctions to collapse when a measurement is made
Correspondence principle – for large orbits and for large energies, quantum calculations must agree with classical calculations. Formulated by Bohr in 1920
The modern theory of antimatter began in 1928, with a paper by Paul Dirac. Dirac realized that his relativistic version of the Schrodinger wave equation for electrons predicted the possibility of antielectrons. These were discovered by Carl D. Anderson in 1932 and named positrons (a contraction of ’positive electrons’)
Dirac equation is a relativistic quantum mechanical wave equation formulated by British physicist Paul Dirac in 1928 which provides a description of elementary spin-½ particles, such as electrons, consistent with both the principles of quantum mechanics and the theory of special relativity. The equation demands the existence of antiparticles and actually predated their experimental discovery, making the discovery of the positron, the antiparticle of the electron, one of the greatest triumphs of modern theoretical physics
Double-slit experiment or Young's experiment involves particle beams or coherent waves passing through two closely-spaced slits, after which in many circumstances they are found to interfere with each other. The light appears as a series of rainbow stripes, known as Young’s fringes. In quantum mechanics the double-slit experiment demonstrates the inseparability of the wave and particle natures of light and other quantum particles
EPR paradox – an early and influential critique leveled against quantum mechanics. Albert Einstein and his colleagues Boris Podolsky and Nathan Rosen (known collectively as EPR) designed a thought experiment intended to reveal what they believed to be inadequacies of quantum mechanics. Disproved by quantum entanglement
Gauge invariance (also called gauge symmetry) is the property of a field theory in which different configurations of the underlying fundamental but unobservable fields result in identical observable quantities. A theory with such a property is called a gauge theory
Gauge symmetry explains why all the particles of a given type are indistinguishable
In quantum mechanics, the Heisenberg uncertainty principle states by precise inequalities that certain pairs of physical properties, like position and momentum, cannot simultaneously be known to arbitrary precision, i.e. the more you know the position of a particle, the less you can know about its velocity, and the more you know about the velocity of a particle, the less you can know about its instantaneous position
Heisenberg uncertainty principle explains how packets of energy can exist for short time periods in a vacuum
In quantum mechanics, the Hamiltonian is the operator corresponding to the total energy of the system
Hidden variable theories were espoused by some physicists who argued that the state of a physical system, as formulated by quantum mechanics, does not give a complete description for the system; i.e., that quantum mechanics is ultimately incomplete, and that a complete theory would provide descriptive categories to account for all observable behaviour and thus avoid any indeterminism. Supported by Louis de Broglie and David Bohm
Bell's theorem states that any physical theory that incorporates local realism, favoured by Einstein, cannot reproduce all the predictions of quantum mechanical theory. A series of experiments by John Bell proved this to be true, ruling out local hidden variable theories
Alain Aspect performed Bell’s inequality experiments, proving conclusively that local hidden variable theories don’t work. Quantum entanglement and faster-than-light communication does happen. Einstein called the idea of entanglement ‘spooky action at a distance’
Lamb shift, named after Willis Lamb, is a small difference in energy between the S and P energy levels of the hydrogen atom in QED
Loop quantum gravity (LQG) is a theory that attempts to describe the quantum properties of gravity. Space can be viewed as an extremely fine fabric or network ‘woven’ of finite loops
Pauli exclusion principle – in its simplest form for electrons in a single atom, it states that no two electrons can have the same four quantum numbers (principal, azimuthal, magnetic and spin). More generally, no two identical fermions (particles with half-integer spin) may occupy the same quantum state simultaneously, i.e. no two electrons can be in the same place with the same properties at the same time. In contrast, integer spin particles, bosons, are not subject to the Pauli exclusion principle
Photoelectric effect – a phenomenon in which electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner are known as photoelectrons
In 1887, Heinrich Hertz discovered that electrodes illuminated with ultraviolet light create electric sparks more easily. In 1905 Albert Einstein published a paper that explained experimental data from the photoelectric effect as being the result of light energy being carried in discrete quantized packets (photons). This discovery led to the quantum revolution
Planck constant (denoted h) is a physical constant reflecting the sizes of quanta in quantum mechanics. The Planck constant was first described as the proportionality constant between the energy (E) of a photon and the frequency of its associated electromagnetic wave (ν). This relation between the energy and frequency is called the Planck relation or the Planck–Einstein equation (E = h ν). In SI units, the Planck constant is expressed in joule seconds (J·s)
Planck's constant = 6.626068 × 10-34 m2 kg / s
Planck units are physical units of measurement defined exclusively in terms of five universal physical constants
Planck length is a unit of length equal to 1.6 x 10−35 metres
Standard deviation of position * standard deviation of momentum ≥ Planck constant / 2
Many-worlds interpretation is an interpretation of quantum mechanics that asserts the objective reality of the universal wavefunction and denies the actuality of wavefunction collapse. Many-worlds implies that all possible alternative histories and futures are real, each representing an actual ‘world’ (or ‘universe’). The original relative state formulation is due to Hugh Everett in 1957. Later, this formulation was popularized and renamed many-worlds by Bryce DeWitt
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925. It extended the Bohr Model by describing how the quantum jumps occur. It did so by interpreting the physical properties of particles as matrices that evolve in time
In 1972 Murray Gell-Mann and Harald Fritzsch introduced the conserved quantum number ’colour charge’, and coined the term quantum chromodynamics (QCD) as the gauge theory of the strong interaction. The quark model is a part of QCD
QCD – quantum chromodynamics: a theory of strong interactions between elementary particles
Quantum decoherence gives the appearance of wave function collapse (the reduction of the physical possibilities into a single possibility as seen by an observer). Decoherence occurs when a system interacts with its environment in a thermodynamically irreversible way. Decoherence can be viewed as the loss of information from a system into the environment
A quantum dot is a nanocrystal made of semiconductor materials that are small enough to display quantum mechanical properties. Quantum dots were discovered by Alexei Ekimov. Researchers have studied applications for quantum dots in transistors, solar cells, LEDs, and diode lasers
QED – quantum electrodynamics: the quantum field theory of the electromagnetic force. It explains how light and matter interact and includes the effects of special relativity
The quantum eraser experiment described in this article is a variation of Thomas Young's classic double-slit experiment. It establishes that when a photon is acted upon in a fashion that allows which slit it has passed through to be determined, the photon cannot interfere with itself. When a stream of photons is marked in this way, then the interference fringes characteristic of the Young experiment will not be seen
Quantum entanglement – a physical phenomenon that occurs when pairs (or groups) of particles are generated or interact in ways such that the quantum state of each member must subsequently be described relative to the other
Quantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parameterized (represented) by an infinite number of dynamical degrees of freedom. It supposes that all fields are carried across space by fundamental particles. Also implied is that particles of any one type are indistinguishable, particles are omitted and absorbed during interactions, and antimatter exists
Quantum friction – the idea that two objects moving past each other experience a friction–like lateral force that arises from quantum fluctuations in the vacuum
Quantum gravity (QG) is the field of theoretical physics attempting to unify quantum mechanics with general relativity in a self-consistent manner. Quantum gravity theory does not work, as quantum mechanics and Einstein’s gravity model do not combine
In quantum mechanics an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state
There are four quantum numbers which can describe the electron completely –
1. principal quantum number describes the electron shell, or energy level, of an atom
2. azimuthal quantum number describes the subshell, and gives the magnitude of the orbital angular momentum
3. magnetic quantum number describes the specific orbital (or ‘cloud’) within that subshell
4. spin projection quantum number describes the spin (intrinsic angular momentum) of the electron within that orbital
Quantum superposition – a fundamental principle of quantum mechanics that holds that a physical system – such as an electron – exists partly in all its particular theoretically possible states simultaneously; but when measured or observed, it gives a result corresponding to only one of the possible configurations
Quantum teleportation – a process by which quantum information (e.g. the exact state of an atom or photon) can be transmitted (exactly, in principle) from one location to another, with the help of classical communication and previously shared quantum entanglement between the sending and receiving location
Quantum tunnelling – the phenomenon where a particle tunnels through a barrier that it classically could not surmount. Tunnelling is often explained using the Heisenberg uncertainty principle and the wave–particle duality of matter. First proposed by Friedrich Hund in 1926. Its first application was a mathematical explanation for alpha decay, which was done in 1928 by George Gamow
Quark–gluon plasma (QGP) is a phase of quantum chromodynamics which is hypothesized to exist at extremely high temperature and/or density. This phase is thought to consist of free quarks and gluons
Rayleigh–Jeans law attempts to describe the spectral radiance of electromagnetic radiation at all wavelengths from a black body at a given temperature. The Rayleigh–Jeans law agrees with experimental results at large wavelengths (or, equivalently, low frequencies) but strongly disagrees at short wavelengths (or high frequencies). This inconsistency between observations and the predictions of classical physics is commonly known as the ultraviolet catastrophe. Max Planck solved the problem by postulating that electromagnetic energy did not follow the classical description, but could only be emitted in discrete packets of energy proportional to the frequency, as given by Planck's law
In quantum mechanics, the Schrodinger equation describes how the quantum state of a physical system changes in time. Published in 1926. Schrodinger expressed the probability of a particle being in a given place at some time in terms of a ‘wavefunction’, we includes all the information we know about that particle
Schrodinger's cat is a thought experiment, often described as a paradox, devised by Austrian physicist Erwin Schrodinger in 1935. It illustrates what he saw as the problem of the Copenhagen interpretation of quantum mechanics applied to everyday objects. The thought experiment presents a cat that might be alive or dead, depending on an earlier random event. A cat is locked in a steel chamber, along with a ‘diabolical device’: a flask of poisonous hydrocyanic acid, to be shattered only upon the decay of a radioactive atom. The cat’s fate depends on the probability of whether the atom decayed or not
String theory attempts to reconcile quantum mechanics and general relativity. String theory posits that the electrons and quarks within an atom are not 0-dimensional objects, but rather 1-dimensional oscillating lines (strings), possessing only the dimension of length, but not height or width. Bosonic string theory requires that there are 26 dimensions
M-theory is an extension of string theory in which 11 dimensions of spacetime are identified as 7 higher-dimensions plus the 4 common dimensions
Superstring theory is an attempt to explain all of the particles and fundamental forces of nature in one theory by modelling them as vibrations of tiny supersymmetric strings. Superstring theory is a shorthand for supersymmetric string theory because unlike bosonic string theory, it is the version of string theory that incorporates fermions and supersymmetry
A membrane, brane, or p-brane is a spatially extended mathematical concept that appears in string theory and related theories (e.g. M-theory and brane cosmology). The membrane exists in a static number of dimensions. The visible, four-dimensional universe is restricted to a brane inside a higher-dimensional space, called the ‘bulk’
Unified field theory allows all that is usually thought of as fundamental forces and elementary particles to be written in terms of a single field. There is no accepted unified field theory
Wave–particle duality is the concept that all matter exhibits both wave and particle properties. Being a central concept of quantum mechanics, this duality addresses the inadequacy of classical concepts like ‘particle’ and ‘wave’ in fully describing the behavior of quantum-scale objects. Proposed by Louis de Broglie
Zero point energy – the lowest possible energy that a quantum mechanical physical system may have and is the energy of the ground state
In quantum mechanics, bra–ket notation is a standard notation for describing quantum states, composed of angle brackets and vertical bars
Thermodynamics
Thermodynamics deals with the relationships and conversions between heat and other forms of energy
Laws of Thermodynamics –
0. The zeroth law of thermodynamics, which underlies the definition of temperature. ‘If two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other’
1. The first law of thermodynamics, which mandates conservation of energy, and states in particular that heat is a form of energy. ‘Energy can be neither created nor destroyed. It can only change forms. In any process in an isolated system, the total energy remains the same. For a thermodynamic cycle the net heat supplied to the system equals the net work done by the system’
2. The second law of thermodynamics, which states that the entropy of the universe always increases, or (equivalently) that perpetual motion machines are impossible. The second law means that heat will tend to be transferred from a hot object to a cold one. ‘Consider two isolated systems in separate but nearby regions of space, each in thermodynamic equilibrium in itself (but not in equilibrium with each other). Then let some event break the isolation that separates the two systems, so that they become able to exchange matter or energy. Wait till the exchanging systems reach mutual thermodynamic equilibrium. Then the sum of the entropies of the initial two isolated systems is less than or equal to the entropy of the final exchanging systems. In the process of reaching a new thermodynamic equilibrium, entropy (S) has increased (or at least has not decreased). Both matter and energy exchanges can contribute to the entropy increase’
Maxwell’s demon is a thought experiment created by James Clerk Maxwell to ‘show that the Second Law of Thermodynamics has only a statistical certainty’. It demonstrates Maxwell's point by hypothetically describing how to violate the Second Law: a container of gas molecules at equilibrium is divided into two parts by an insulated wall, with a door that can be opened and closed by Maxwell's demon. The demon opens the door to allow only the faster than average molecules to flow through to a favored side of the chamber, and only the slower than average molecules to the other side, causing the favoured side to gradually heat up while the other side cools down, thus decreasing entropy
3. The third law of thermodynamics, which concerns the entropy of an object at absolute zero temperature, and implies that it is impossible to cool a system all the way to exactly absolute zero. Atoms still have a very small amount of motion due to zero-point energy. ‘As temperature approaches absolute zero, the entropy of a system approaches a constant minimum’
Enthalpy (symbol H) – a thermodynamic function of a system, equivalent to the sum of the internal energy of the system plus the product of its volume multiplied by the pressure exerted on it by its surroundings. The amount of thermal energy contained in a system – heat content. H = U + P*V
∆H = Q (heat) if pressure is constant
Standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy from the formation of 1 mole of the compound from its constituent elements, with all substances in their standard states at 101.3 kPa and 298 K. A negative value indicates that the reaction is exothermic
Enthalpy of fusion (heat of fusion) – the change in enthalpy resulting from heating one mole of a substance to change its state from a solid to a liquid. The temperature at which this occurs is the melting point
Enthalpy of vaporization, also known as the (latent) heat of vaporization or heat of evaporation, is the enthalpy change required to transform a given quantity of a substance from a liquid into a gas at a given pressure
Hess’s law states that the total enthalpy change during the complete course of a reaction is the same whether the reaction is made in one step or in several steps
Entropy (symbol S) – a measure of how organized or disorganized a system is. The extent to which the energy of a system is available for conversion to work
Entropy is the only quantity in the physical sciences that requires a particular direction for time, sometimes called an ‘arrow of time’. As one goes ‘forward’ in time, the second law of thermodynamics says, the entropy of an isolated system will increase
Rudolf Clausius is considered one of the central founders of the science of thermodynamics. His most important paper, On the mechanical theory of heat, published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy
Gas laws
Amagat's law – or the Law of Partial Volumes describes the behaviour and properties of mixtures of ideal (as well as some cases of non-ideal) gases
Avogadro’s Law – equal volumes of gases at the same temperature and pressure contain the same number of molecules regardless of their chemical nature and physical properties
Boyle’s law – for a given mass, at constant temperature, the pressure times the volume is a constant, pV = C
Charles’s law – at constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature (in kelvins) increases or decreases. Formula is V/T = k
Dalton's law (also called Dalton's law of partial pressures) states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture. This empirical law was observed by John Dalton in 1801 and is related to the ideal gas laws
Gay-Lussac's law, also known as the law of combining volumes, is actually two laws. Gay-Lussac's law states that the ratio between the combining volumes of gases and the product, if gaseous, can be expressed in small whole numbers, which Gay-Lussac discovered in 1809. In 1811, Avogadro used Gay-Lussac's data to form Avogadro's hypothesis (equal volumes of gases at the same temperature and pressure contain the same number of molecules) which later gave way to modern gas stoichiometry. The other law (the pressure law), discovered in 1802, states that the pressure of a fixed amount of gas at fixed volume is directly proportional to its temperature in kelvins. It is expressed mathematically as P/T = k
Graham’s Law – the rate of effusion of a gas is inversely proportional to the square root of the mass of its particles
Henry’s law – the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid
Three earlier gas laws – Boyle's law (1662), Charles' law (1787–1802), and Gay-Lussac's law (1809), were combined to form the combined gas law, which with the addition of Avogadro's law later gave way to the ideal gas law: PV = nRT wher P is pressure, V is volume, n is the amount of gas in moles, R is the gas constant and T is absolute temperature
Boltzmann's equation is a probability equation relating the entropy S of an ideal gas to the quantity W, which is the number of microstates corresponding to a given macrostate:
S = k ln W where k is Boltzmann constant equal to 1.3806 x 10−23 joule/kelvin
S = k log W is on Boltzmann’s grave in Vienna
Boltzmann constant – gas constant R divided by the Avogadro constant NA, k = R/NA
It has the same dimension (energy divided by temperature) as entropy
Effusion – the process in which individual molecules flow through a hole without collisions between molecules
Gas constant – equivalent to the Boltzmann constant, but expressed in units of energy (i.e. the pressure-volume product) per kelvin per mole (rather than energy per kelvin per particle). Its value is 8.3145 Jmol-1K-1
Ideal gas – a theoretical gas composed of a set of randomly moving, non-interacting point particles
Maxwell–Boltzmann distribution is a probability distribution with applications in physics and chemistry. The most common application is in the field of statistical mechanics. The temperature of any (massive) physical system is the result of the motions of the molecules and atoms which make up the system. The Maxwell–Boltzmann distribution applies to the classical ideal gas
Molar volume – the volume occupied by one mole of an ideal gas
Heat – the process of energy transfer from one body or system to another due to thermal contact
Heat can be transferred by conduction, convection and radiation
Conduction – a cool object is warmed up by coming into contact with a hot object
Convection – the transfer of heat in a liquid or gas by means of a current that circulates as a result of temperature differences
Radiation – the transfer of heat through space. It represents a conversion of thermal energy into electromagnetic energy
Heat capacity or thermal capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount. Heat capacity is expressed in units of joules per kelvin. The molar heat capacity is the heat capacity per mole of a pure substance and the specific heat capacity, often simply called specific heat, is the heat capacity per unit mass of a material
Specific heat capacity – the heat capacity per unit mass of a material
Adiabatic – without loss or gain of heat
Thermal conductivity – the property of a material's ability to conduct heat
Thermal diffusivity – thermal conductivity divided by the volumetric heat capacity. Substances with high thermal diffusivity rapidly adjust their temperature to that of their surroundings
Thermal energy – the form of energy that a substance possesses by virtue of the movement of its molecules and atoms
Thermal equilibrium – achieved when two systems in thermal contact with each other cease to have a net exchange of energy. It follows that if two systems are in thermal equilibrium, then their temperatures are the same
James Joule studied the nature of heat, and discovered its relationship to mechanical work. This led to the theory of conservation of energy, which led to the development of the first law of thermodynamics. The SI unit of energy, the joule, is named after him. He worked with Lord Kelvin to develop the absolute scale of temperature, made observations on magnetostriction, and found the relationship between the flow of current through a resistance and the heat dissipated, now called Joule's law
Joule's first law shows the relation between heat generated by an electric current flowing through a conductor. Q = I2Rt where Q is the amount of heat, I is the electric current flowing through a conductor, R is the amount of electric resistance present in the conductor, and t is the amount of time that this happens for
Joule's second law says that the internal energy of a gas does not change if volume and pressure change, but does change if temperature changes
Joule’s gravestone is inscribed with the number 772.55, his climacteric 1878 measurement of the mechanical equivalent of heat
Temperature – a measurement of the average kinetic energy of the molecules in an object or system
Kelvin temperature scale – Absolute zero, or 0°K, is the temperature at which molecular energy is a minimum, and it corresponds to a temperature of -273.15°C. Water freezes at 273 K and boils at 373 K
Rankine temperature scale – Absolute zero, or 0°R, is the temperature at which molecular energy is a minimum. Zero on both the Kelvin and Rankine scales is absolute zero, but the Rankine degree is defined as equal to one degree Fahrenheit, rather than the one degree Celsius used by the Kelvin scale. A temperature of −459.67 °F is exactly equal to 0 °R
Reaumur scale is a temperature scale in which the freezing and boiling points of water are set to 0 and 80 degrees respectively
Newton’s law of cooling simply states that the temperature of a hot (or cold) object progresses toward the temperature of its environment in a simple exponential fashion
Resistance thermometers are temperature sensors that exploit the predictable change in electrical resistance of some materials with changing temperature. Made of platinum
Carnot Cycle – a thermodynamic cycle proposed by Sadi Carnot in 1824. It is the most efficient existing cycle capable of converting a given amount of thermal energy into work
Carnot heat engine – a hypothetical engine that operates on the reversible Carnot cycle
When the Carnot cycle is plotted on a pressure volume diagram, the isothermal stages follow the isotherm lines for the working fluid, adiabatic stages move between isotherms and the area bounded by the complete cycle path represents the total work that can be done during one cycle
Carnot heat-engine cycle is a totally reversible cycle. If all the processes that comprise it are reversed, it becomes the Carnot refrigeration cycle
Critical point – also called critical state, specifies the conditions (temperature, pressure) at which the liquid state of the matter ceases to exist
Curie point – or Curie temperature) the temperature above which a substance loses its magnetism
Triple point – the temperature and pressure at which three phases (gas, liquid, and solid) of that substance may coexist in thermodynamic equilibrium. For example, the triple point temperature of mercury is at -38.83 °C, at a pressure of 0.2 mPa. The triple point of water is used to define the kelvin, the SI unit of thermodynamic temperature
Gibbs free energy – a thermodynamic potential that measures the ‘usefulness’ or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure. Used to determine whether a reaction will be spontaneous
Helmholtz free energy – a thermodynamic potential that measures the ‘useful’ work obtainable from a closed thermodynamic system at a constant temperature
Isothermal process – a change of a system, in which the temperature remains constant
Microstate – a specific microscopic configuration of a thermodynamic system that the system may occupy with a certain probability in the course of its thermal fluctuations. In contrast, the macrostate of a system refers to its macroscopic properties, such as its temperature and pressure
Quasistatic process – a thermodynamic process that happens infinitely slowly. A quasistatic process ensures that the system will go through a sequence of states that are infinitesimally close to equilibrium (so the system remains in quasistatic equilibrium), in which case the process is typically reversible
A reversible process can be ‘reversed’ by means of infinitesimal changes in some property of the system without entropy production (i.e. dissipation of energy). Any reversible process is necessarily a quasistatic one. However, some quasistatic processes are irreversible, e.g. a compression against a system with a piston subject to friction
State variable – is one of the set of variables that are used to describe the mathematical ‘state’ of a dynamical system. In a thermodynamic system, properties such as temperature, pressure, volume, internal energy, enthalpy, and entropy are state variables
Stefan-Boltzmann constant – a physical constant denoted by the Greek letter σ (sigma), is the constant of proportionality in the Stefan–Boltzmann law: the total energy radiated per unit surface area of a black body in unit time is proportional to the fourth power of the thermodynamic temperature
Thermoelectric effect – the direct conversion of temperature differences to electric voltage and vice-versa. Discovered by Thomas Seebeck in 1821
Electricity and magnetism
Electricity – the set of physical phenomena associated with the presence and flow of electric charge
Alternating current – the direction of flow changes at regular intervals. The voltage moves between large positive and negative values
Alternator – an electromechanical device that converts mechanical energy to electrical energy in the form of alternating current
Armature – a revolving structure in an electric motor or generator, wound with the coils that carry the current
An electric battery is a device consisting of one or more electrochemical cells that convert stored chemical energy into electrical energy. Each battery consists of a negative electrode material, a positive electrode material, an electrolyte that allows ions to move between the electrodes, and terminals that allow current to flow out of the battery to perform work
Baghdad Battery – the common name for a number of artifacts created in Mesopotamia, during the Parthian or Sassanid periods. They may have been galvanic cells, perhaps used for electroplating gold onto silver objects
Leclanche cell – has carbon (positive) and zinc (negative) terminals
Lithium batteries are long-life batteries
Lithium-ion batteries are rechargeable
Nickel–cadmium battery (NiCad battery) is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes
A wet cell battery (such as a lead-acid car battery) contains a liquid electrolyte. It is often rechargeable. A dry cell (such as a flashlight battery) does not. The electrolyte is usually a moist paste however, so it is not completely ‘dry’. It may or may not be rechargeable
Capacitance – the ratio of charge to potential on an electrically charged, isolated conductor. Measured in farads. A 1 farad capacitor when charged with 1 coulomb of electrical charge will have a potential difference of 1 volt between its plates
Charge = current x time
Conductor – a material that allows heat or electrical energy to pass through it
Coulomb’s law – the magnitude of the electrostatic force between two point electric charges (F) is directly proportional to the product of the magnitudes of each of the charges (q1 and q2) and inversely proportional to the square of the distance between the two charges (r). F = Ke (q1 * q2) / r2 where Ke is Coulomb’s constant 9 *109 Nm2C-2
Commutator – the moving part of a rotary electrical switch in certain types of electric motors or electrical generators that periodically reverses the current direction between the rotor and the external circuit
An electric current in a wire creates a circular magnetic field around the wire, its direction (clockwise or counter-clockwise) depending on that of the current.
A current is induced in a loop of wire when it is moved towards or away from a magnetic field, or a magnet is moved towards or away from it, the direction of current depending on that of the movement
Two wires with current flowing in the same direction will attract each other
Two wires with current flowing in the opposite direction will repel each other
Daniell cell – voltaic cell with constant voltage
Dielectric – an electrical insulator that can be polarized by an applied electric field
Direct current – flows in one direction only and has a constant voltage. Produced by batteries and solar cells
Dynamo – an electrical generator that produces direct current with the use of a commutator
Electric charge – a property of some subatomic particles, which determines their electromagnetic interactions. Electrically charged matter is influenced by, and produces, electromagnetic fields. Differing charges attract one another, while like charges repel
Electric current – a movement or flow of electrically charged particles, typically measured in amperes
An electrical circuit is a path which electrons from a voltage or current source follow. Electric current flows in a closed path called an electric circuit
Electric field – a region in which there is an electric charge. Any charged particle entering the region experiences a force. Describes the electric force experienced by a motionless positively electrically charged test particle at any point in space relative to the source of the field. An electric field has units of Force/Charge (Newtons / Coulomb)
Electric potential – the capacity of an electric field to do work on an electric charge, typically measured in volts
Electric potential energy – total work
The electrical resistance of an electrical element is the opposition to the passage of an electric current through that element; the inverse quantity is electrical conductance, the ease at which an electric current passes
Electrodynamics – electric charges that are flowing in a current
Electrostatics – electric charges that are at rest
Faraday constant – the magnitude of electric charge per mole of electrons
Faraday's laws of electrolysis are quantitative relationships based on the electrochemical researches published by Michael Faraday in 1834
Gain – a measure of the ability of a circuit (often an amplifier) to increase the power or amplitude of a signal from the input to the output
Induction – the act or process by which an electric or magnetic effect is produced in an electrical conductor or magnetizable body when it is exposed to the influence or variation of a field of force
Leiden Jar – the first electrical capacitor, stores static electricity. Invented in 1745 in the Dutch city of Leiden
Lenz’s law – An induced current is always in such a direction as to oppose the motion or change causing it
Load cell – a transducer that is used to convert a force into an electrical signal. Used in several types of measuring instruments
Ohm’s law – V = IR, where V is the potential difference measured across the resistance in units of volts; I is the current through the resistance in units of amperes and R is the resistance of the conductor in units of ohms
Piezoelectricity – the ability of some materials (notably crystals and certain ceramics, including bone) to generate an electric potential in response to applied mechanical stress. The first demonstration of the direct piezoelectric effect was in 1880 by the brothers Pierre Curie and Jacques Curie
Polyphase system is a means of distributing alternating current electrical power. Polyphase systems have three or more energized electrical conductors carrying alternating currents with a definite time offset between the voltage waves in each conductor. Allow alternating current to be transmitted economically over long distances
Potential difference – the difference in electrical charge between two points in a circuit expressed in volts
Potentiometer – a three-terminal resistor with a sliding contact that forms an adjustable voltage divider. If only two terminals are used (one side and the wiper), it acts as a variable resistor or rheostat
Rectifier – device that converts AC to DC
Resistance – opposition of a circuit to the flow of electric current
Short circuit – a low-resistance connection established by accident or intention between two points in an electric circuit
Solenoid – a loop of wire, often wrapped around a metallic core, which produces a magnetic field when an electric current is passed through it. Solenoids are important because they can create controlled magnetic fields and can be used as electromagnets
Static electricity – the build up of electric charge on the surface of objects. The static charges remain on an object until they either bleed off to ground or are quickly neutralized by a discharge
Telluric current – an electric current which moves underground or through the sea
Tesla coil – an electrical resonant transformer circuit invented by Nikola Tesla around 1891. It is used to produce high-voltage, low-current, high frequency alternating-current electricity
Thermionic emission is the flow of electrons from a metal or metal oxide surface
Transformer – a device used to transfer electric energy from one circuit to another, especially a pair of multiply wound, inductively coupled wire coils that effect such a transfer with a change in voltage, current, phase, or other electric characteristic
The transformer is based on two principles: firstly, that an electric current can produce a magnetic field (electromagnetism), and, secondly that a changing magnetic field within a coil of wire induces a voltage across the ends of the coil (electromagnetic induction). Changing the current in the primary coil changes the magnetic flux that is developed. The changing magnetic flux induces a voltage in the secondary coil
For a transformer, the ratio of the voltages is the same as the ratio of the turns in the coils
Triboelectricity – static electricity produced by friction
Van de Graaff generator is an electrostatic generator which uses a moving belt to accumulate very high electrostatically stable voltages on a hollow metal globe on the top of the stand. Invented in 1929, the potential differences achieved in modern Van de Graaff generators can reach 5 megavolts
Volt – named after the Italian scientist Count Alessandro Volta, a 19th Century nobleman who invented the electric battery
Voltage (potential difference) = current x resistance
In 1800, as the result of a professional disagreement over the galvanic response advocated by Galvani, Volta invented the voltaic pile, an early electric battery, which produced a steady electric current
Wimshurst machine – an electrostatic generator, a machine for generating high voltages developed between 1880 and 1883 by British inventor James Wimshurst
Magnetism, at its root, arises from two sources: 1) Electric currents or more generally, moving electric charges create magnetic fields; 2) Many particles have nonzero ‘intrinsic’ (or ‘spin’) magnetic moments. Just as each particle, by its nature, has a certain mass and charge, each has a certain magnetic moment, possibly zero
There are four types of magnetism –
1. Diamagnetism – appears in all materials, and is the tendency of a material to oppose an applied magnetic field, and therefore, to be repelled by a magnetic field
2. Paramagnetism – in a paramagnetic material there are unpaired electrons. An unpaired electron is free to align its magnetic moment in any direction. When an external magnetic field is applied, these magnetic moments will tend to align themselves in the same direction as the applied field, thus reinforcing it
3. Ferromagnetism – a ferromagnet, like a paramagnetic substance, has unpaired electrons. However, in addition to the electrons' intrinsic magnetic moment's tendency to be parallel to an applied field, there is also in these materials a tendency for these magnetic moments to orient parallel to each other to maintain a lowered energy state. Thus, even when the applied field is removed, the electrons in the material maintain a parallel orientation. Iron, nickel and cobalt are ferromagnetic. The basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. Strongest type of magnetism. Iron, cobalt and nickel are the only ferromagnetic elements
4. Ferrimagnetism – ferrimagnetic materials include the mineral magnetite and also have unpaired electrons. The spins of the electrons tend to line up in opposite directions but the spin is greater in one direction than the other, and the overall effect still produces magnetism
Magnetic domain describes a region within a magnetic material which has uniform magnetization
Magnetic reluctance or ‘magnetic resistance’, is analogous to resistance in an electrical circuit
Any change in a magnetic field produces a force
Tesla – SI unit of magnetic flux density (the strength of a magnetic field)
Weber – SI unit of magnetic flux (a measure of the strength and extent of a magnetic field)
Magnetic flux is the dot product of the magnetic field and the area vectors
Oersted (abbreviated as Oe) is the CGS unit of magnetizing field (also known as magnetic field strength or intensity). Oersted is most widely known for observing that electric currents induce magnetic fields
Nuclear magnetic resonance (NMR) is a property that magnetic nuclei have in a magnetic field and applied electromagnetic (EM) pulse or pulses, which cause the nuclei to absorb energy from the EM pulse and radiate this energy back out. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI)
Electromagnetism – magnetism produced by electric charge in motion
Electromagnetic radiation is produced when electrons and positrons collide
Electromagnetic induction is the production of voltage across a conductor moving through a magnetic field
The electromagnetic interaction occurs between charged subatomic particles, and is carried by photons
In electromagnetism, permeability is the measure of the ability of a material to support the formation of a magnetic field within itself, i.e. it is the degree of magnetization that a material obtains in response to an applied magnetic field
Electromagnetic radiation – radiation consisting of waves of energy associated with electric and magnetic fields resulting from the acceleration of an electric charge
Electrophoresis – the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field
Faraday discovered electromagnetic induction in 1831 and demonstrated the dynamo
Faraday’s law of induction – the induced electromotive force (EMF) in any closed circuit is equal to the time rate of change of the magnetic flux through the circuit
The EMF generated by Faraday's law of induction due to relative movement of a circuit and a magnetic field is the phenomenon underlying electrical generators, electrical motors and transformers
Heinrich Hertz expanded the electromagnetic theory of light that had been put forth by Maxwell. He was the first to satisfactorily demonstrate the existence of electromagnetic waves by building an apparatus to produce and detect VHF or UHF radio waves
Inductance is the ability of an inductor to store energy in a magnetic field
Insulator – a material such as glass or porcelain with negligible electrical or thermal conductivity
Lorentz force – the combination of electric and magnetic force on a point charge due to electromagnetic fields. If a particle of charge q moves with velocity v in the presence of an electric field E and a magnetic field B, then it will experience a force F = q (E + v x B)
James Clerk Maxwell mathematically predicted the existence of electromagnetic waves of diverse wavelengths, but he died in 1879 before his prediction was experimentally verified. Oliver Lodge demonstrated the existence of Maxwell’s waves transmitted along wires in 1887–88. Heinrich Hertz showed experimentally, in 1888, the existence of electromagnetic waves in free space. Subsequently, Lodge pursued Hertz’s work and delivered a commemorative lecture in June 1894 (after Hertz’s death)
Maxwell's equations are a set of four partial differential equations that relate the electric and magnetic fields to their sources, charge density and current density. These equations can be combined to show that light is an electromagnetic wave
1. Also known as Gauss’s law, describes the electric field around a charged object
2. Magnetic field lines are always closed loops; there is no such thing as a magnetic monopole
3. Varying currents cause magnetic fields (induction). Generalization of Faraday’s law
4. Varying magnetic fields create currents (induction)
Oersted observed that electric currents induce magnetic fields
Electromagnetic spectrum – the range of all possible frequencies of electromagnetic radiation
Far-red light – longest wavelength subdivision of infrared
Infrared (IR) radiation is electromagnetic radiation of a wavelength longer than that of visible light, but shorter than that of radio waves
The existence of infrared radiation was first discovered in 1800 by astronomer William Herschel, who called it ‘calorific rays’
Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than soft X-rays
Ultraviolet radiation was discovered by Johann Ritter
Wavelengths in electromagnetic spectrum (m) – Gamma Ray (10-12), X-ray (10-10), Ultraviolet (10-8), Visible (.5x10-6), Infrared (10-5), Microwave (10-2), Radio (103)
VHF – frequency 30 – 300 MHz, wavelength 10m – 1m
UHF – frequency 300 MHz – 3 GHz, wavelength 1m – 10cm
FM radio broadcasts use frequencies in the VHF band
AM radio is divided into three frequency ranges – long wave (153 – 279 kHz), medium wave (531 – 1620 kHz) and short wave (2.31 – 25.82 MHz)
UK television is broadcast in the UHF radio band
Red light – long wavelength (620–750 nm)
Violet light – short wavelength (380–450 nm)
Microwaves can be produced by a magnetron, which passes electrons through electrical and magnetic fields to make them spin, causing cavities in the magnetron to resonate at microwave frequencies
The existence of radio waves was predicted by James Clerk Maxwell in 1864 from his equations. In 1888, Heinrich Hertz was the first to demonstrate the existence of radio waves by building a spark gap radio transmitter that produced 450 MHz microwaves
Microwave ovens use electromagnetic radiation to cause vibrations in the water molecules contained in food. The energy given to the water by the microwaves then spreads through the food as heat
Infrared radiation can be classified as near, intermediate or far, according to how far it is from the red end of the visible spectrum
Visible light – 380 nm (violet) to 780 nm (red)
Ultraviolet radiation can be classified as near, middle or extreme, according to how far it is from the violet end of the visible spectrum
X-rays are produced when high-speed electrons strike a solid target. X-rays from about 10 to 0.10 nm wavelength are classified as ‘soft’ X-rays, and from about 0.10 to 0.01 nm wavelength as ‘hard’ X-rays, due to their penetrating abilities. Hard X-rays can penetrate solid objects, and their largest use is to take images of the inside of objects in diagnostic radiography and crystallography
The discovery of UV radiation was associated with the observation that silver salts darkened when exposed to sunlight. In 1801, the German physicist Johann Ritter made the observation that invisible rays just beyond the violet end of the visible spectrum darkened silver chloride-soaked paper more quickly than violet light itself
Antenna gain is the ratio of the power density of an antenna's radiation pattern in the direction of strongest radiation to that of a reference antenna
Blueshift – any decrease in wavelength (increase in frequency); the opposite of redshift. In visible light, this shifts the colour from the red end of the spectrum to the blue end. Blueshift is most commonly caused by relative motion toward the observer, described by the Doppler effect
Redshift – happens when light seen coming from an object is proportionally shifted to appear ‘redder’. Here, the term redder refers to what happens when visible light is shifted toward the red end of the visible spectrum. More generally, where an observer detects electromagnetic radiation outside the visible spectrum, ‘redder’ amounts to a technical shorthand for ‘increased in electromagnetic wavelength’. Occurs when the object is moving away from the observer
Black body – an object that absorbs all electromagnetic radiation that falls onto it. No radiation passes through it and none is reflected. A black body emits a temperature-dependent spectrum of light. This thermal radiation from a black body is termed black-body radiation
Permeability – the measure of the ability of a material to support the formation of a magnetic field within itself
Relativity
Special relativity is a theory of the structure of spacetime. It was introduced in Einstein's 1905 paper On the Electrodynamics of Moving Bodies. Special relativity is based on two postulates which are contradictory in classical mechanics:
1. The laws of physics are the same for all observers in uniform motion relative to one another (principle of relativity)
2. The speed of light in a vacuum is the same for all observers, regardless of their relative motion or of the motion of the light source
‘The faster you travel, the slower time moves’ – special theory of relativity (1905)
Near light speed, objects become heavier and shorter and time slows
E=mc2. c is celeritas (Latin for ‘speed) and represents the speed of light. Mass–energy equivalence arose originally from special relativity, as developed by Albert Einstein, who proposed this equivalence in 1905
Speed of light – 300,000 km per second (186,000 miles per second)
Speed of light is approximately 1 foot per nanosecond
Lorentz transformation – describes how, according to the theory of special relativity, two observers' varying measurements of space and time can be converted into each other's frames of reference
Henri Poincare introduced the modern principle of relativity and was the first to present the Lorentz transformations in their modern symmetrical form
In 1971 four atomic clocks were flown round the world. The moving clocks each lost a fraction of a second compared with an identical clock on the ground, confirming Einstein’s Special Theory of Relativity
Michelson–Morley experiment was performed in 1887 by Albert Michelson and Edward Morley. Its results are generally considered to be the first strong evidence against the theory of a luminiferous aether. An interferometer was used to show the constancy of the speed of light across multiple inertial frames. The negative results initiated a line of research that eventually led to special relativity
General relativity is a theory of gravitation developed by Einstein in the years 1907–1915. General Relativity is needed because Einstein’s special theory of relativity conflicts with Newton’s law of gravitation
‘Gravity is caused by the curvature of time and space’ – general theory of relativity (1915)
In Einstein's theory of general relativity, the Schwarzschild solution (or the Schwarzschild vacuum) describes the gravitational field outside a spherical, non-rotating mass such as a (non-rotating) star, planet, or black hole
Arthur Eddington wrote a number of articles which announced and explained Einstein's theory of general relativity
Following his research on general relativity, Einstein entered into a series of attempts to generalize his geometric theory of gravitation to include electromagnetism as another aspect of a single entity. In 1950, he described his ‘unified field theory’ in a Scientific American article
Time dilation is a phenomenon described by the theory of relativity. It can be illustrated by supposing that two observers are in motion relative to each other, or differently situated with regard to nearby gravitational masses. They each carry a clock of identical construction and function. Then, the point of view of each observer will generally be that the other observer's clock is in error
Gravitational waves are theoretical ripples in the curvature of spacetime which propagate as a wave, traveling outward from the source. Laser Interferometer Gravitational-Wave Observatory (LIGO) is a large-scale experiment aiming to directly detect gravitational waves
Wheeler–DeWitt equation is an attempt to mathematically meld the ideas of quantum mechanics and general relativity, a step toward a theory of quantum gravity. In this approach, time plays no role in the equation
Bernhard Riemann was the first to suggest using dimensions higher than merely three or four in order to describe physical reality, an idea that was ultimately vindicated by Einstein
Wormhole – officially known as an Einstein–Rosen bridge, is a hypothetical topological feature of spacetime that would fundamentally be a shortcut through spacetime. A wormhole is much like a tunnel with two ends, each in separate points in spacetime. Lorentzian wormholes known as Schwarzschild wormholes or Einstein-Rosen bridges are bridges between areas of space that can be modeled as vacuum solutions to the Einstein field equations
Classical mechanics
Acceleration = change in velocity / time taken for change
A = (V-U)/T, where A is acceleration, V is final velocity, U is initial velocity and T is time
Angular momentum – the amount of rotation an object has, taking into account its mass and shape. It is a vector quantity that represents the product of a body's rotational inertia and rotational velocity about a particular axis
Angular momentum = angular velocity x moment of inertia
Law of conservation of angular momentum states that when no external torque acts on an object or a closed system of objects, no change of angular momentum can occur
Angular velocity – the rate of change of angular displacement. A vector quantity which specifies the angular speed (rotational speed) of an object and the axis about which the object is rotating. The SI unit of angular velocity is radians per second, and is usually represented by the symbol lower-caase omega (ω)
Boiling point – the temperature at which the vapour pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapour
Boiling point of water is 212o F
Brittle – having little elasticity; hence easily cracked or fractured or snapped
Bulk modulus of a substance measures the substance's resistance to uniform compression
Cam – converts circular to linear motion
The Cavendish experiment, performed in 1797–1798 by Henry Cavendish, was the first experiment to measure the force of gravity between masses in the laboratory and the first to yield accurate values for the gravitational constant and the mass of the Earth
Centrifugal force – the apparent force, equal and opposite to the centripetal force, drawing a rotating body away from the centre of rotation, caused by the inertia of the body
Centrifugal force = mass x centripetal acceleration
This force is also sometimes written in terms of the angular velocity ω of the object about the centre of the circle: F = mr ω2
Centripetal accelertion = v2/r
Centripetal force – the inward force on a body moving in a curved path around another body
Coefficient of friction – a dimensionless scalar value which describes the ratio of the force of friction between two bodies and the force pressing them together
Coefficient of restitution (COR) of two colliding objects is a fractional value representing the ratio of speeds after and before an impact, taken along the line of the impact
Damping – an influence within or upon an oscillatory system that has the effect of reducing, restricting or preventing its oscillations
Density – the amount of mass per unit of volume. Measured in kg/m3
Density of water = 1000 kg/m3 at 4oC
Air density decreases with increasing altitude, as does air pressure. It also changes with variances in temperature or humidity. At sea level and at 15 °C, air has a density of approximately 1.225 kg/m3
Relative density – ratio of the density of a substance to the density of a standard substance at the same temperature
Displacement = time x average velocity (assuming constant acceleration)
Ductility – the ability of a material to be elongated in tension
Elasticity – the tendency of a body to return to its original shape after it has been stretched or compressed
Energy – the ability to do work
Thomas Young was the first to define the term ‘energy’ in the modern sense
Work done = force x distance moved in direction of force
Energy transformed = potential difference x charge
Electrical energy = voltage x current x time
Efficiency = (energy usefully transferred / total energy supplied) x 100
Energy density – the amount of energy stored in a given system or region of space per unit volume or mass
Equilibrium – a condition in which all acting influences are canceled by others, resulting in a stable, balanced, or unchanging system
Escape velocity – the speed at which the kinetic energy plus the gravitational potential energy of an object is zero. It is the speed needed to ‘break free’ from the gravitational attraction of a massive body, without further propulsion. For a spherically symmetric body, the escape velocity at a given distance is calculated by the formula v = √ (2Gm/r) where G is the universal gravitational constant, M the mass of the planet, and r the distance from the centre of gravity
To leave planet Earth, an escape velocity of 11.2 km/s (approx. 40,320 km/h, or 25,000 mph) is required
Force – any influence that changes the speed or direction of movement of a body
Resultant force = mass x acceleration
Force = change in momentum / time taken for change
Gravitational field – the area around a body within which any other body will experience a force of gravitational attraction
Gravity – the force of attraction that exists between objects because of their masses
g = 9.81 m/s2 (mass of earth / radius of earth2)
Hamiltonian mechanics was first formulated by William Rowan Hamilton in 1833, starting from Lagrangian mechanics, a previous reformulation of classical mechanics introduced by Joseph Louis Lagrange in 1788
Hooke's law of elasticity is an approximation that states that the amount by which a material body (a spring) is deformed (the strain) is linearly related to the force causing the deformation (the stress) F = kx (force = stiffness constant x displacement)
Impulse – a force multiplied by the amount of time it acts over, i.e. change in momentum
Inertia – the tendency of an object in motion to remain in motion, or an object at rest to remain at rest, unless acted upon by a force. The property of matter to resist acceleration or deceleration, i.e. any motion which is not in a straight line and with constant velocity
Inverse-square law – any physical law stating that a specified physical quantity or strength is inversely proportional to the square of the distance from the source of that physical quantity
Kerma – an acronym for ‘kinetic energy released per unit mass’, defined as the sum of the initial kinetic energies of all the charged particles liberated by uncharged ionizing radiation. The SI unit of kerma is the gray
Kinetic energy – the energy possessed by a body because of its motion. E = ½mv2 where E is energy (in joules), m is mass, and v is velocity
Kinematics – the study of classical mechanics which describes the motion of points, bodies (objects) and systems of bodies (groups of objects) without consideration of the causes of motion
Mach number – the speed of an object moving through air, or any other fluid substance, divided by the speed of sound
Six classical simple machines – lever, wheel and axle, pulley, inclined plane, wedge, and screw
Malleability – capable of being shaped or formed, as by hammering or pressure
Mass – a measure of the amount of matter contained in a body. Remains constant when gravity changes. Measured in kilograms
Mechanics – the analysis of the action of forces on matter or material
Mechanical advantage – a measure of the force amplification achieved by using a tool, mechanical device or machine system, e.g. a lever
Moment – the perpendicular distance from a point to a line or a surface
Moment of force – the product of the distance of a force from an axis times the magnitude of the force
Moment of inertia – the mass property of a rigid body that defines the torque needed for a desired change in angular velocity about an axis of rotation
Momentum = mass x velocity
Newton’s Laws of Motion
First law – A body remains at rest or moves with constant velocity in a straight line unless acted on by an external force. Law of Inertia (first described by Galileo)
Second law – The rate of change of momentum of an object is proportional to the force applied to it, and the change takes place in the direction of the applied force. Force = mass x acceleration, F = ma
Third law – Every action has an equal and opposite reaction
Newton's law of universal gravitation (1687) states that every massive particle in the universe attracts every other massive particle with a force which is directly proportional to the product of their masses and inversely proportional to the square of the distance between them F = G (m1 x m2) / d2
Newtonian constant of gravitation (G) 6.6742 x 10-11 Nm2kg-2
Newton’s law of universal gravitation was confirmed by the return of Halley’s comet
Normal force – the component, perpendicular to the surface (surface being a plane) of contact, of the contact force exerted on an object by, for example, the surface of a floor or wall, preventing the object from penetrating the surface
Poisson’s ratio – the negative ratio of transverse to axial strain
Potential energy – the mechanical energy that a body has by virtue of its position; stored energy
Gravitational potential energy – the potential energy associated with gravitational force. Change in gravitational potential energy = mass x gravitational field strength x height difference
Elastic potential energy – the potential energy of an elastic object (for example a bow or a catapult) that is deformed under tension or compression
Chemical potential energy – a form of potential energy related to the structural arrangement of atoms or molecules
Electrical potential energy – an object can have potential energy by virtue of its electric charge and several forces related to their presence
Gravitational potential energy – work is required to elevate objects against Earth's gravity
Power – the rate at which work is performed or energy is converted. For electrical energy, power (watts) = volts x amps
Power = work done (energy transformed) / time
Precession – a change in the orientation of the rotational axis of a rotating body
Pressure – the ratio of force to the area over which that force is distributed
Standard atmospheric pressure – 101325 Pa
Pressure volume diagram (or PV diagram) is used to describe corresponding changes in volume and pressure in a system. PV diagrams, originally called indicator diagrams, were developed in the 18th century as tools for understanding the efficiency of steam engines
Principle of least action – the motion between two points takes place in such a way that the action has a minimum value with reference to all other paths between the points which correspond to the same energy, i.e. the most economical route will be taken
Pycnometer – device used to determine the density of a liquid
Resonance – the tendency of a system to oscillate with larger amplitude at some frequencies than at others
Retrograde motion is in the direction opposite to the movement of something else
Rheology – the study of materials with both solid and fluid characteristics
Simple harmonic motion – a type of periodic motion where the restoring force is directly proportional to the displacement. It can serve as a mathematical model of a variety of motions, such as the oscillation of a spring, or the motion of a simple pendulum. Simple harmonic motion is typified by the motion of a mass on a spring when it is subject to the linear elastic restoring force given by Hooke's Law
Sonics – the study of mechanical vibrations in matter
Specific gravity – the ratio of the density of a material to the density of water
Speed = distance / time
Speed of light in vacuum, commonly denoted c, is exactly 299,792,458 metres per second, a figure that is exact because the length of the metre is defined from this constant and the international standard for time. This is approximately 186,282 miles per second, or about 671 million miles per hour
Stiffness is the rigidity of an object – the extent to which it resists deformation in response to an applied force. The complementary concept is flexibility or pliability
Strain – a description of deformation in terms of relative displacement of particles in the body
Stress – a measure of the internal forces acting within a deformable body
Surface tension – a contractive tendency of the surface of a liquid that allows it to resist an external force
Tension – the pulling force exerted by a string or cable on another object. It results from the net electrostatic attraction between the particles in a solid when it is deformed so that the particles are further apart from each other than when at equilibrium. Measured in Newtons parallel to the string on which it applies
Tension is the opposite of compression
A free-falling object achieves its terminal velocity when the downward force of gravity equals the upward force of drag. The terminal velocity of a human is 120 mph
Thrust – a reaction force described quantitatively by Newton's second and third laws. When a system expels or accelerates mass in one direction the accelerated mass will cause a proportional but opposite force on that system
Torque – the tendency of a force to rotate an object about an axis, fulcrum, or pivot
Torque (T) = radial distance (r) x rotational force (F). Cross product
Anticlockwise movement is positive torque, clockwise movement is negative torque
Velocity – a vector measurement of the rate and direction of motion
Velocity = displacement / time
Final velocity = initial velocity + (acceleration x time)
Weight – a measure of the force exerted on a body by a gravitational field. Measured in newtons
Weight = mass x gravitational field strength
Work – a form of energy. Energy transferred by force. Work done equals force times distance, W = Fd
Yield strength or yield point of a material is defined as the stress at which a material begins to deform plastically. Prior to the yield point the material will deform elastically and will return to its original shape when the applied stress is removed
Young's Modulus (E) (also known as the modulus of elasticity) is a measure of the stiffness of a given material. It is defined as the ratio, for small strains, of the rate of change of stress with strain
Fluid mechanics
Fluid – a substance in which atoms and molecules move freely past each other. Anything that takes the shape of its container, e.g. liquids and gases
Archimedes’ Principle – any object, wholly or partially immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object. More tersely: buoyancy = weight of displaced fluid
In fluid dynamics, Bernoulli's principle states that for an inviscid flow, an increase in the speed of the fluid occurs simultaneously with a decrease in pressure or a decrease in the fluid's potential energy. Bernoulli's principle is named after the Dutch-Swiss mathematician Daniel Bernoulli who published his principle in his book Hydrodynamica in 1738. Fundamental to the design of aircraft wings
Bernoulli’s equation – velocity2/2 + gravity*height + pressure/density = constant
Boundary layer – the layer of fluid in the immediate vicinity of a bounding surface where the effects of viscosity are significant. In the Earth's atmosphere, the planetary boundary layer is the air layer near the ground affected by diurnal heat, moisture or momentum transfer to or from the surface. On an aircraft wing the boundary layer is the part of the flow close to the wing, where viscous forces distort the surrounding non-viscous flow
Capillary action – the ability of a liquid to flow in narrow spaces without the assistance of, and in opposition to external forces like gravity
Hydraulics – the physical science and technology of the static and dynamic behavior of fluids
Hydrostatics – the science of fluids at rest
Hydrostatic equilibrium or hydrostatic balance occurs when compression due to gravity is balanced by a pressure gradient force in the opposite direction
Inviscid flow – the flow of an ideal fluid that is assumed to have no viscosity
Magnetohydrodynamics – the dynamics of electrically conducting fluids. Examples of such fluids include plasmas, liquid metals, and salt water or electrolytes
Navier-Stokes equations, named after Claude-Louis Navier and George Gabriel Stokes, describe the motion of fluid substances
In fluid mechanics, the Reynolds number Re is a dimensionless number that gives a measure of the ratio of inertial forces to viscous forces
Skin friction – drag on a body moving through a fluid
In fluid dynamics an object is moving at its terminal velocity if its speed is constant due to the restraining force exerted by the air, water or other fluid through which it is moving. Turbulence or turbulent flow – type of fluid (gas or liquid) flow in which the fluid undergoes irregular fluctuations, or mixing, in contrast to laminar flow, in which the fluid moves in smooth paths or layers
Upthrust – a force that pushes an object upwards when it is in a fluid (a liquid or gas). Also known as buoyancy
Venturi effect – the reduction in fluid pressure that results when a fluid flows through a constricted section of pipe. The Venturi effect is named after Giovanni Battista Venturi
The Venturi effect is a jet effect; as with a funnel the velocity of the fluid increases as the cross sectional area decreases, with the static pressure correspondingly decreasing. According to the laws governing fluid dynamics, a fluid's velocity must increase as it passes through a constriction to satisfy the principle of continuity, while its pressure must decrease to satisfy the principle of conservation of mechanical energy. Thus any gain in kinetic energy a fluid may accrue due to its increased velocity through a constriction is negated by a drop in pressure
Viscosity – resistance to flow, caused by internal friction
Optics
Ambient light – the natural light in a scene
Anamorphic projection – a modification of the aspect ratio of an image by optical distortion which stretches or compresses the image in one dimension but not the other
Angle of incidence – a measure of deviation of something from ‘straight on’, for example in the approach of a ray to a surface
Chromatic aberration – a type of distortion in which there is a failure of a lens to focus all colours to the same convergence point. It occurs because lenses have a different refractive index for different wavelengths of light (the dispersion of the lens). The refractive index decreases with increasing wavelength
Collimator – a device that narrows a beam of particles or waves
Diffraction – the apparent bending and spreading of waves when they meet an obstruction. The effect is most notable when the gap is the same size as the wavelength
Diffraction grating – an optical component with a periodic structure, which splits and diffracts light into several beams travelling in different directions. The directions of these beams depend on the spacing of the grating and the wavelength of the light
Dispersion – separation of visible light into its various colours when it passes between media of different density, e.g. air and glass, when white light passes through a prism
Fermat's principle or the principle of least time is the idea that the path taken between two points by a ray of light is the path that can be traversed in the least time. This principle is sometimes taken as the definition of a ray of light
Incandescence – the emission of light from a hot body due to its temperature
Incandescent light bulb produces light by heating a filament wire to a high temperature until it glows
Incident light – light falling on a surface, not the light reflected from it
Laser – any of several devices that emit highly amplified and coherent radiation of one or more discrete frequencies. One of the most common lasers makes use of atoms in a metastable energy state that, as they decay to a lower energy level, stimulate others to decay, resulting in a cascade of emitted radiation. Lasers differ from other sources of light because they emit light coherently
When the additive primaries (red, green and blue) are mixed in equal proportions, they produce white light
When the subtractive primaries (cyan, magenta and yellow) are mixed in equal proportions, they produce black
Light cone – the path that a flash of light, emanating from a single event and traveling in all directions, would take through spacetime
Luminiferous ether – term used in 19th century to describe a medium for the propagation of light. Explained how light could travel in a vacuum
Luminescense – the emission of light that does not derive energy from the temperature of the emitting body
Lumen – SI unit of luminous flux; the measure of the perceived power of light
Lux – SI unit of illuminance; the total luminous flux incident on a surface, per unit area. It is a measure of the intensity of the incident light (brightness)
Maser – a device a device that produces coherent electromagnetic waves through amplification by stimulated emission. Historically, maser derives from the original, upper-case acronym MASER, which stands for ‘Microwave Amplification by Stimulated Emission of Radiation’. The lower-case usage arose from technological development having rendered the original denotation imprecise, because contemporary masers emit EM waves (microwave and radio frequencies). Charles Townes, J. P. Gordon, and H. J. Zeiger built the first maser, independently, at Columbia University in 1953
Mean free path of a particle – the average distance covered by a particle (photon, atom or molecule) between subsequent impacts
Any incoming ray that is parallel to the axis of a parabolic mirror will be reflected to a central point, or focus. The distance from the mirror to the focus is the focal length. The distance from the mirror to twice the focal length is the centre of curvature
Newton’s rings – an interference pattern caused by the reflection of light between two surfaces – a spherical surface and an adjacent flat surface
Newton’s experiments on light discredited the ideas of Descartes that white light was pure
In the mid-1660s, Isaac Newton bought a pair of prisms at a fair near Cambridge, which were to be the basis of a series of experiments that would unlock a secret that had occupied scientists for centuries – the nature of light itself. To explain what he had done, Newton created a diagram. It is called The Crucial Experiment. Newton’s diagram demonstrated that light is not pure, but made up of different colours, as the different colours are refracted by different amounts. Newton's famous experiment with two prisms demonstrates that a prism does not alter the colour of the light, but is merely separating the colours that are already present
Newton thought that light was compose of tiny particles called corpuscles
Optical rotation (optical activity) is the turning of the plane of linearly polarized light about the direction of motion as the light travels through certain materials
Two periodic waveforms whose phase difference is ¼ of their output period are said to have a quadrature phase relationship
Phot – a unit of illumination equal to 1 lumen per square centimeter; 10,000 phots equal 1 lux
Polarization – a process or state in which rays of light exhibit different properties in different directions, especially the state in which all the vibration takes place in one plane
The lenses of polarized sunglasses reduce glare reflected at some angles off shiny non-metallic surfaces such as water
Rayleigh scattering, named after the British physicist Lord Rayleigh, is the elastic scattering of light or other electromagnetic radiation by particles much smaller than the wavelength of the light. Rayleigh scattering causes the blue hue of the daytime sky and the reddening of the sun at sunset
Real image – an image in which the outgoing rays from a point on the object pass through a single point. It is easiest to observe real images when projected on an opaque screen
Reflections can be divided into two types: specular reflection and diffuse reflection
Specular reflection – the mirror-like reflection of light from a surface, in which light from a single incoming direction (a ray) is reflected into a single outgoing direction. Such behavior is described by the law of reflection, which states that the direction of incoming light (the incident ray), and the direction of outgoing light reflected (the reflected ray) make the same angle with respect to the surface normal, thus the angle of incidence equals the angle of reflection
Diffuse reflection – the reflection of light from a surface such that an incident ray is reflected at many angles rather than at just one angle as in the case of specular reflection
Total internal reflection – a phenomenon that happens when a propagating wave strikes a medium boundary at an angle larger than a particular critical angle with respect to the normal to the surface. If the refractive index is lower on the other side of the boundary and the incident angle is greater than the critical angle, the wave cannot pass through and is entirely reflected
Refraction occurs when light travels through an area of space that has a changing index of refraction; this principle allows for lenses and the focusing of light
Refractive index – a number that describes how light, or any other radiation, propagates through that medium. Its most elementary occurrence is in Snell's law of refraction
Snell’s law is a formula used to describe the relationship between the angles of incidence and refraction, when referring to light or other waves passing through a boundary between two different isotropic media, such as water and glass
Scintillation – a flash of light produced in a transparent material by the passage of a particle (an electron, an alpha particle, an ion, or a high-energy photon)
Spherical aberration – an optical effect observed in an optical device that occurs due to the increased refraction of light rays when they strike a lens or a reflection of light rays when they strike a mirror near its edge, in comparison with those that strike nearer the centre
Galilean telescope used a convergent (plano-convex) objective lens and a divergent (plano-concave) eyepiece lens. A Galilean telescope, because the design has no intermediary focus, results in a non-inverted and upright image. Galileo’s best telescope magnified objects about 30 times. Designed in Venice in 1609
A refracting or refractor telescope is a type of optical telescope that uses a lens as its objective to form an image
A reflecting telescope (also called a reflector) is an optical telescope which uses a single or combination of curved mirrors that reflect light and form an image
Tyndall effect is an effect of light scattering by colloidal particles or particles in suspension. It is named after the 19th century Irish scientist John Tyndall
Virtual image – an image in which the outgoing rays from a point on the object always diverge. It will appear to converge in or behind the optical device (e.g. a mirror)
Waves
Wave – a vibration that transfers energy from one place to another
A harmonic of a wave is a component frequency of the signal that is an integer multiple of the fundamental frequency
Higher frequency waves have a shorter wavelength. Frequency (in hertz) = speed / wavelength
Longitudinal waves – waves that have the same direction of vibration along their direction of travel, which means that the vibration of the medium (particle) is in the same direction or opposite direction as the motion of the wave. Also known as compression waves
Transverse waves – waves that consist of oscillations occurring perpendicular to the direction of energy transfer, e.g. waves that travel along a string
Interference – the addition of two or more waves that results in a new wave pattern
Constructive interference – waves are in phase
Destructive interference – waves are out of phase
Non-sinusoidal waveforms are waveforms that are not pure sine waves, e.g. square waves, rectangular waves
Period of a wave – length of cycle measured in seconds. Inverse of frequency
Velocity of a wave = wavelength / period
A standing wave – also known as a stationary wave – is a wave that remains in a constant position
Wave speed = frequency x wavelength
The hotter the body, the shorter the wavelength. Hot gases near the Sun can emit X-rays; some extremely hot stars emit gamma rays
Rarefaction – the reduction of a medium's density, or the opposite of compression. Air at higher layers of the atmosphere is less dense, or is rarefied, in relation to air at lower layers
Reflection – the change in direction of a wavefront at an interface between two different media so that the wavefront returns into the medium from which it originated
Refraction – the change in direction of a wave due to a change in its speed. This is most commonly observed when a wave passes from one medium to another at an angle. Waves with short wavelengths are refracted more than those with long wavelengths, e.g. blue light is refracted more than red light
Evanescent wave – a wave with an intensity that exhibits exponential decay without absorption as a function of the distance from the boundary at which the wave was formed, e.g. energy from light that passes through a mirror
Fundamental frequency – the lowest frequency of a periodic waveform
Interferometry – a family of techniques in which waves, usually electromagnetic, are superimposed in order to extract information about the waves
Michelson interferometer is the most common configuration for optical interferometry and was invented by Albert Abraham Michelson
Doppler effect – the apparent change of frequency of light or sound because of the relative motion of the source and the observer. It is commonly heard when a vehicle sounding a siren or horn approaches, passes, and recedes from an observer. Compared to the emitted frequency, the received frequency is higher during the approach, identical at the instant of passing by, and lower during the recession
Modulation is the process of varying one or more properties of a high-frequency periodic waveform, called the carrier signal, with a modulating signal which typically contains information to be transmitted
Sound – a vibration that propagates as a typically audible mechanical wave of pressure and displacement
White, pink, blue, purple and brown – types of noise
White Noise – random noise that has uniform power spectral density at every frequency in the range of interest
Speed of sound is 768 mph
Speed of sound c is given by the Newton-Laplace equation c = √ (K/p) where K is a coefficient of stiffness and p is the density
Sound does not travel in a vacuum
A sonic boom is the sound associated with the shock waves created by an object traveling through the air faster than the speed of sound
Infrasound – sound that is lower in frequency than 20 Hz (Hertz) or cycles per second, the normal limit of human hearing
Ultrasound – sound that is higher in frequency than 20,000 Hz, the normal limit of human hearing. Used to obtain images for medical diagnostic purposes
States of matter
Quarks and leptons together make up ‘ordinary matter’, and their interactions contribute to the effective volume of the composite particles that make up ordinary matter. Matter commonly exists in four states (or phases); solid, liquid, gas, and plasma. However, advances in experimental techniques have revealed other previously theoretical phases, such as Bose–Einstein condensates and fermionic condensates
Solid – holds a rigid shape without a container. Atoms or molecules vibrate around a fixed point
Electronic band structure of a solid describes those ranges of energy that an electron within the solid may have (called energy bands, allowed bands, or simply bands), and ranges of energy that it may not have (called band gaps or forbidden bands)
For many solids dissolved in liquid water, the solubility increases with temperature up to 100 °C
Liquid – a mostly non-compressible fluid. Atoms or molecules do not have the fixed positions they have in a solid, nor do they have the freedom of movement they have in a gas
Gas – a compressible fluid. Atoms or molecules constantly move in a random way
The word ‘gas’ is a neologism first used by the 17th century Flemish chemist J.B. van Helmont
Plasma – a collection of charged particles that respond strongly and collectively to electromagnetic fields, taking the form of gas-like clouds or ion beams
Bose–Einstein condensate is a state of matter formed by bosons cooled to temperatures very near to absolute zero. Under such conditions, a large fraction of the bosons occupy the lowest quantum state. In 1995 the first gaseous condensate was produced by Eric Cornell and Carl Wieman at the University of Colorado, using a gas of rubidium atoms cooled to 170 nanokelvin
Fermionic condensate is a superfluid phase formed by fermionic particles at low temperatures. Produced for the first time by Deborah Jin in 2003
Josephson Effect is the phenomenon of supercurrent across a device known as a Josephson junction, and consisting in two superconductors coupled by a weak link (normally a thin layer of insulator)
Meissner effect – the expulsion of a magnetic field from a superconductor during its transition to the superconducting state. The superconductor asks like a magnetic mirror, and a magnet can float about the superconductor’s surface (magnetic levitation)
Mpemba effect – the assertion that warmer water can sometimes freeze faster than colder water. Named after the Tanzanian schoolboy who discovered it
Phase boundary – boundary between two phases of physical matter
A phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas
Phase transitions
The solid-to-liquid transition is called melting
The liquid-to-solid transition is called freezing
The liquid-to-gas transition is called vaporisation (boiling / evaporation)
The gas-to-liquid transition is called condensation
The solid-to-gas transition is called sublimation
The gas-to-solid transition is called deposition
Liquefaction – the conversion of a solid or a gas into a liquid
Sublimation – used to purify iodine. The sublimation of solid carbon dioxide (dry ice) produces smoke effects
The gas-to-plasma transition is called ionization
The plasma-to-gas transition is called recombination
Regelation – the phenomenon of melting under pressure and freezing again when the pressure is reduced
Superconductivity – property of particular metals at extremely low temperatures. The electrical resistance of a conductor becomes zero, so that an electric current can flow without loss
Superconductivity is a phenomenon of exactly zero electrical resistance and expulsion of magnetic fields occurring in certain materials when cooled below a characteristic critical temperature. It was discovered by Heike Onnes in 1911 in Leiden
The complete microscopic theory of superconductivity was finally proposed in 1957 by Bardeen, Cooper, and Schrieffer. Independently, the superconductivity phenomenon was explained by Nikolay Bogolyubov. This BCS theory explained the superconducting current as a superfluid of Cooper pairs, pairs of electrons interacting through the exchange of phonons. Superconductivity breaks down if the electrons acquire enough energy to jump the band gap. The band gap scales with the critical temperature (the temperature below which resistance vanishes)
Cooper pair – two electrons (or other fermions) that are bound together at low temperatures in a certain manner. The Cooper pair state is responsible for superconductivity
Supercritical fluid – any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist
Supercurrent – a superconducting current, that is, electric current which flows without dissipation
Superfluidity is a phase of matter characterized by the complete absence of viscosity
A supersolid is a spatially ordered material (that is, a solid or crystal) with superfluid properties
Superfluidity was originally discovered in liquid helium. In liquid helium-4, the superfluidity occurs at far higher temperatures than it does in helium-3. Each atom of helium-4 is a boson particle, by virtue of its zero spin. A helium-3 atom, however, is a fermion; it can form bosons only by pairing with itself at much lower temperatures
Vapour – a substance in the gas phase at a temperature lower than its critical point
Spectroscopy
Spectroscopy was originally the study of the interaction between radiation and matter as a function of wavelength (λ). Historically, spectroscopy referred to the use of visible light dispersed according to its wavelength, e.g. by a prism. Later the concept was expanded greatly to comprise any measurement of a quantity as a function of either wavelength or frequency. Gustav Kirchhoff formalized three laws of spectroscopy
Anders Angstrom was one of the founders of spectroscopy. The ångström unit (1 Å = 10-10 m) with which the lengths on a scale of the wavelength of light or interatomic spacings in condensed matter is measured are named for him. The unit is used in crystallography as well as spectroscopy
Emission spectrum – the spectrum of frequencies of electromagnetic radiation emitted due to an atom's electrons making a transition from a high energy state to a lower energy state
Emission spectrum of atomic hydrogen is divided into a number of spectral series named after scientists, with wavelengths given by the Rydberg formula. The first series (transition to the first shell, n=1), is named after Theodore Lyman
Fraunhofer lines are a set of spectral lines named for the German physicist Joseph von Fraunhofer. The lines were originally observed as dark features (absorption lines) in the optical spectrum of the Sun. Each absorption line corresponds to a particular chemical element seen in various states and energies
D-line is one of the classified Fraunhofer lines observed in the visible spectrum of the Sun's electromagnetic radiation. Sodium vapour in the upper layers of the Sun creates a dark line in the emitted spectrum of electromagnetic radiation
Spectral line – a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used as a sort of ‘atomic fingerprint,’ as gases emit light at very specific frequencies when exposed to electromagnetic waves, which are displayed in the form of spectral lines. When electrons drop to a lower energy level (lose energy) they give off a bright emission line (a photon). When electrons jump to a higher energy level (gain energy) they give produce dark absorption lines
Spectrometer (spectrograph or spectroscope) – an instrument used to measure properties of light over a specific portion of the electromagnetic spectrum, typically used in spectroscopic analysis to identify materials
Spectrum – an array of entities, as light waves or particles, ordered in accordance with the magnitudes of a common physical property, as wavelength or mass
Stark effect – the shifting and splitting of spectral lines of atoms and molecules due to the presence of an external static electric field
Zeeman Effect – the effect of splitting a spectral line into several components in the presence of a static magnetic field, similar to the action of a prism on white light. It is analogous to the Stark effect
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation
Fluorescence – luminescence in which light of a visible color is emitted from a substance under stimulation or excitation by light or other forms of electromagnetic radiation or by certain other means. The light is given off only while the stimulation continues; in this the phenomenon differs from phosphorescence, in which light continues to be emitted after the excitation by other radiation has ceased
Fluorescence is derived from the word fluorspar, which glows in ultraviolet light
Commonly seen examples of phosphorescent materials are the glow-in-the-dark toys, paint, and clock dials that glow for some time after being charged with a bright light
Thermoluminescence – a form of luminescence that is exhibited by certain crystalline materials, such as some minerals, when previously absorbed energy from electromagnetic radiation or other ionizing radiation is re-emitted as light upon heating of the material
Thermoluminescence dating – the determination by means of measuring the accumulated radiation dose of the time elapsed since material containing crystalline minerals was either heated (lava, ceramics) or exposed to sunlight (sediments)
SI Units
For information on SI Units see Weights and Measures